The standard treatment of tuberculosis (TB) that is susceptible to first-line anti-TB drugs, the most common form of the disease, currently recommended by all international and national guidelines,1–5 is based on the administration of a combination of isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E) in the initial phase of 2 months, followed by the combination of isoniazid and rifampicin (2HRZE/4HR) in a 4-month continuation phase. The recommendation is to administer the drugs in a fixed-dose combination, that is to say, all together in the same presentation; using the drugs separately in different presentations is not recommended in order to avoid a lack of therapeutic adherence and the consequent risk of emergence of resistance, and to facilitate compliance, since the administration of each drug separately involves taking a greater number of pills.
Drug-resistant TB is far more difficult to treat and cure, particularly when at least rifampicin and isoniazid are involved (known in this case as multi-drug resistant [MDR]-TB),5 since it requires longer treatment with medications that are less common, more expensive, and less effective. Another risk of MDR-TB is that it can be transmitted to other people, causing difficulties in disease control.
It should be noted that in Spain the incidence of MDR-TB, at a rate of 1.9%,6 is low in comparison with other countries, mainly due to the fact that the disease has always been treated by experienced doctors using fixed-dose combination drugs.
Between December 2018 and May 2019, drugs used in fixed-dose combinations were in short supply in Spain, in particular preparations containing isoniazid, rifampicin and pyrazinamide, and rifampin plus isoniazid. Unavailability of the combination of rifampicin and isoniazid means that drugs have to be prescribed in different, individual preparations, i.e. separately, with the risk of monotherapy occurring if the patient stops taking one and continues to take the other, a situation in which the tuberculosis bacillus can develop permanent and irreversible resistance.
During this same period, there were also shortages of the parenteral form of rifampicin, a basic drug in TB treatment that is needed for the intravenous treatment of severe cases who cannot receive it by mouth. This shortage, therefore, represents a significant risk in the treatment of the patients.
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), on the initiative of the Tuberculosis and Respiratory Infections (TIR) Area and the Integrated Research Program in Tuberculosis (PII-TB), sent out a questionnaire to all members of the Society in order to determine the impact of these shortages on the management and treatment of their patients.
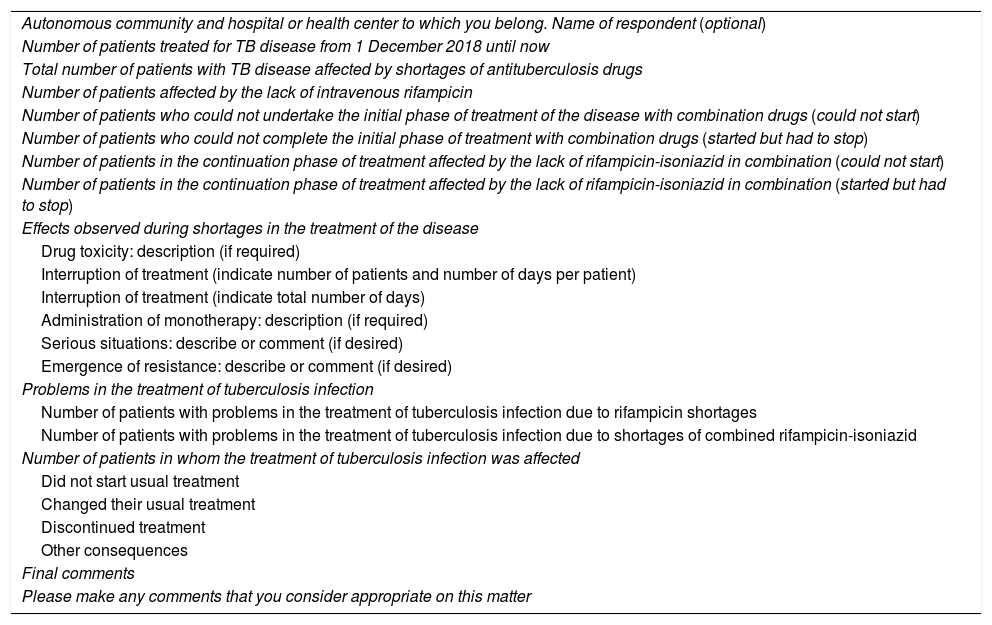
Members completed the questionnaire (Table 1) between 24 May and 8 July 2019. Questions were asked about the number of patients treated during the shortages, the number of patients requiring changes in the treatment of TB and tuberculosis infection due to drug shortages, and the consequences of these shortfalls. Respondents were also offered the possibility of making a personal assessment of the situation.
Survey on shortages of antituberculous drugs.
| Autonomous community and hospital or health center to which you belong. Name of respondent (optional) |
| Number of patients treated for TB disease from 1 December 2018 until now |
| Total number of patients with TB disease affected by shortages of antituberculosis drugs |
| Number of patients affected by the lack of intravenous rifampicin |
| Number of patients who could not undertake the initial phase of treatment of the disease with combination drugs (could not start) |
| Number of patients who could not complete the initial phase of treatment with combination drugs (started but had to stop) |
| Number of patients in the continuation phase of treatment affected by the lack of rifampicin-isoniazid in combination (could not start) |
| Number of patients in the continuation phase of treatment affected by the lack of rifampicin-isoniazid in combination (started but had to stop) |
| Effects observed during shortages in the treatment of the disease |
| Drug toxicity: description (if required) |
| Interruption of treatment (indicate number of patients and number of days per patient) |
| Interruption of treatment (indicate total number of days) |
| Administration of monotherapy: description (if required) |
| Serious situations: describe or comment (if desired) |
| Emergence of resistance: describe or comment (if desired) |
| Problems in the treatment of tuberculosis infection |
| Number of patients with problems in the treatment of tuberculosis infection due to rifampicin shortages |
| Number of patients with problems in the treatment of tuberculosis infection due to shortages of combined rifampicin-isoniazid |
| Number of patients in whom the treatment of tuberculosis infection was affected |
| Did not start usual treatment |
| Changed their usual treatment |
| Discontinued treatment |
| Other consequences |
| Final comments |
| Please make any comments that you consider appropriate on this matter |
A total of 84 questionnaires were returned from 16 autonomous communities and 2 autonomous cities, with the exception of the Balearic Islands. Data were reported on 1,007 patients treated for tuberculosis between December 2018 and the date the questionnaire was completed. The treatment of 550 (54.6%) patients was affected by the shortages. One hundred patients (9.9%) had begun the intensive treatment phase with combination therapy, but could not complete it, and had to switch to individual drugs. In 286 patients (28.4%), the continuation phase of the treatment could not begin with combination therapy, and 248 (24.6%) individuals had to discontinue combination therapy after starting the continuation phase. In 61 patients (6.1%), treatment was interrupted (median 6 days, range 29, 1–30), until the switch to separate drugs was implemented. Seven patients received short periods of monotherapy.
Another part of the survey referred to the influence of the shortage of the rifampicin plus isoniazid combination, which is used in the treatment of tuberculosis infection (infected person without tuberculosis disease). Respondents reported 220 infected individuals who had experienced problems in carrying out their treatment: 77 (35%) failed to start the usual treatment of infection, 116 (53%) switched their initial treatment to an alternative treatment, 9 (4%) stopped treatment, and the problem was not specified in 18 patients (8%).
In their comments on drug shortages and their consequences, respondents mentioned particularly: the gravity of the situation, generally accompanied with the hope that it does not happen again; problems and confusion on the part of patients; the increase in the number of consultations in primary care and hospital clinics; the increase in the activity of both community and hospital pharmacies that are compelled to try to contact other pharmacies in Spain and abroad to try to procure the drugs; and the incredulity of the patients.
Fortunately, no problems occurred in any patient related with the shortage of intravenous rifampicin.
The survey may have limitations, in that it does not represent all patients treated for tuberculosis in Spain (based on the annual incidence of the disease and the period of time analyzed, it probably accounts for approximately 40–45% of the population). However, it does represent virtually all Spanish autonomous communities and a significant number of patients. Its strength is that it reflects the problems caused by the drug shortages, and the concerns expressed by SEPAR members that led this society to embark on negotiations with the health authorities and the pharmaceutical company responsible for the shortfall in production of the preparations in order to solve the problem, and to publish a press release.7
In conclusion, we believe that the shortage of fixed-dose combination anti-antituberculous drugs is a serious problem for healthcare and public health that must be prevented, and any repetition of these circumstances must be strenuously avoided in the future.
We are grateful to all the members of SEPAR who responded to the survey for their indispensable collaboration. We are also grateful for the collaboration of the SEPAR Secretariat in the conduct of the survey, and Mr. Quim Obrador in particular.
Please cite this article as: García-García J-M, et al. Desabastecimiento de fármacos antituberculosos en combinación en España. Arch Bronconeumol. 2020;56:118–119.