Lung transplantation has become in recent years a therapeutic option for infants with terminal lung disease with similar results to transplantation in adults. In Spain, since 1996 114 children lung transplants have been performed; this corresponds to 3.9% of the total transplant number. The most common indication in children is cystic fibrosis, which represents between 70-80% of the transplants performed in adolescents. In infants common indications are interstitial lung disease and pulmonary hypertension. In most children a sequential double lung transplant is performed, generally with the help of extracorpo-real circulation. Lung transplantation in children presents special challenges in monitoring and follow-up, especially in infants, given the difficulty in assessing lung function and performing transbronchial biopsies.There are some more specific complications in children like postransplant lymphoproliferative syndrome or a greater severity of respiratory virus infections. After lung transplantation children usually experiment a very important improvement in their quality of life. Eighty eight per cent of children have no limitations in their activity after 3 years of transplantation. According to the registry of the International Society for Heart & Lung Transplantation (ISHLT) survival at 5 years of transplantation is 54% and at 10 years is around 35%.
El trasplante pulmonar se ha consolidado en los últimos años como una opción terapéutica para los niños con una enfermedad pulmonar terminal con unos resultados similares a los del trasplante en adultos.
En España, desde 1996 se han realizado en menores de 16 años 114 trasplantes, lo que corresponde a un 3,9% del total de trasplantes.
La indicación más frecuente en niños es la fibrosis quística, que representa entre el 70-80% de los trasplantes realizados en adolescentes. En los niños pequeños las indicaciones más frecuentes son las neumopatías intersticiales y la hipertensión pulmonar.
En la mayoría de los niños se realiza un trasplante bipulmonar secuencial, en general con la ayuda de circulación extracorpórea.
El trasplante pulmonar en niños presenta retos especiales en la monitorización y seguimiento, especialmente en los lactantes, dada la dificultad para valorar la función pulmonar y realizar biopsias transbronquiales.
Existen algunas complicaciones más específicas de los niños que de los adultos como el síndrome linfoproliferativo postrasplante o una mayor gravedad de las infecciones por virus respiratorios.
Tras el trasplante pulmonar se produce una mejoría muy importante en la calidad de vida de los niños trasplantados. El 88% de los niños no tienen ninguna limitación en su actividad a los 3 años del trasplante. Según los datos del registro de la International Society for Heart & Lung Transplantation (ISHLT) la supervivencia a los 5 años del trasplante es del 54% y a los 10 años del 30-38%.
In recent years, lung transplantation has become established as a therapeutic option for children with severe lung disease in whom other therapeutic options have failed, resulting in good quality of life and prolonged survival, similar to that of transplants performed in adults.1
Pediatric transplantation has numerous unique features in relation to transplantation in adults2: specific characteristics of children at different stages of development (from infants to adolescents), different indications for different age groups, the effects of immunosuppressant treatments, special susceptibility to infections related to an immature immune system, and particular problems in the availability of suitable donors.
Although pediatric transplant results have improved considerably in the last two decades3 due to advances in the transplant technique, organ preservation, peri-operative management, immunosuppression, and the prophylaxis and treatment of infectious complications, as in adults, chronic graft dysfunction limits long-term survival.
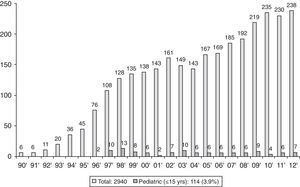
Epidemiology of Pediatric TransplantThe first transplant in an adult in Spain was carried out in 1990. Six transplants were performed that year, with the number of annual transplants increasing gradually to 238 in 2012, to a total of 2940 [Spanish National Transplant Organization (ONT) data].4,5 The ONT considers pediatric transplants as those performed in children under the age of 16. The first pediatric lung transplant was carried out in 1996.6 Since then, 114 pediatric transplants have been performed in Spain, accounting for 3.9% of the total.4 Unlike adult transplants, the annual number of transplants has remained relatively steady, with a median of 6 per year during this period (Fig. 1). In the Spanish Lung Transplant Registry, 74% of children were aged between 11 and 15 years old.5 These data are comparable with those recorded in the registry of the International Society for Heart & Lung Transplantation (ISHLT),1,7 in which pediatric transplantation is considered to be a transplant carried out in children aged under 18. According to ISHLT data, 49,673 lung and heart-lung transplants were performed between 1987 and June 2012, 2542 (5.12%) of which were pediatric. The highest percentage of transplants took place in the adolescent group (12–17 years), representing 84% of pediatric transplants in Europe and 68% in North America.1 In this registry, a very modest increase in the number of pediatric transplants was observed from the 73 carried out in 2000 to 107 in 2011, peaking at 125 transplants in 2010.1
The low frequency of transplants in children and absence of an increase similar to that in adult patients is related to 3 factors8:
- -
The low prevalence of severe lung disease in children.
- -
Advances in the medical care of patients with cystic fibrosis9 (the primary indication in pediatric patients) have considerably improved its course and delayed the age at which lung transplantation may be required.
- -
The limited availability of young donors.
Only a small number of hospitals worldwide carry out pediatric lung transplantation (around 40), and most (88.3%) perform between 1 and 4 transplants per year only, with just 3–4 centers performing between 5 and 9 transplants and 2 centers between 10 and 19 transplants.1 As in Spain, two types of centers can be distinguished among these: centers specifically dedicated to pediatric transplantation, in which transplants are performed in children of all ages (or in some centers in children above preschool age), and adult lung transplant centers in which adolescents or children over 8–10 years of age are also treated.10
IndicationsAs in adults, the general indication for lung transplantation is progressive respiratory failure with a short life expectancy, generally less than 1–2 years, and very poor quality of life.2,11 It requires that all other therapeutic measures have been exhausted, there is no other serious illness, the ability to comply with a complex therapeutic regimen has been assessed, there is a suitable social environment and the family and/or the child assumes the risks of the transplant.
Table 1 specifies the main diseases in which lung transplantation may be indicated in children. Cystic fibrosis is the most common indication; the other major groups of indications are pulmonary vascular diseases (mainly pulmonary hypertension) and diffuse parenchymal lung diseases.12–16
Indications for Lung Transplantation in Children.
| Cystic fibrosis |
| Pulmonary vascular diseases |
| Pulmonary hypertension |
| Other pulmonary vascular disease |
| Pulmonary vein stenosis |
| Alveolar capillary dysplasia |
| Diffuse parenchymal lung diseases |
| Interstitial lung diseases specific to children |
| Surfactant protein deficiency |
| Chronic pneumonitis of infancy |
| Alveolar development disorders |
| Bronchopulmonary dysplasia |
| Post lymphoma pulmonary fibrosis |
| Other interstitial lung diseases |
| Bronchiolitis obliterans after hematopoietic stem cell transplantation |
| Post-infectious bronchiolitis obliterans |
| Pulmonary graft dysfunction (retransplantation) |
In the Spanish Lung Transplant Registry (2006–2010),5 cystic fibrosis accounted for 67.75% of transplants in children aged under 16, post-infectious bronchiolitis obliterans for 12.95%, pulmonary arterial hypertension for 3.2% and interstitial lung diseases, 16.1%. Table 2 shows the number of pediatric transplants carried out in our hospital during the period 1996–2012, with the relative frequency of the different indications.
Indications for Lung Transplant in Children in Hospital Universitari Vall d’Hebron (1996–2012; n=51).
| No., % | |
| Cystic fibrosis | 28 (54.9%) |
| Pulmonary vascular diseases | 8 (15.7%) |
| Idiopathic pulmonary hypertension | 3 |
| Other pulmonary vascular disease | 5 |
| Interstitial disease | 9 (17.7%) |
| Interstitial pneumonitis of infancya | 4 |
| Idiopathic pulmonary fibrosis | 2 |
| Pulmonary fibrosis post-HSCT | 2 |
| Post-lymphoma pulmonary fibrosis | 1 |
| Post-infectious bronchiolitis obliterans | 4 (7.8%) |
| Post-lung transplantation bronchiolitis obliterans syndrome | 2 (3.9%) |
No., number of cases. HSCT, hematopoietic stem cell transplantation.
The proportion of the different indications in relation to age groups has changed in recent years. In the ISHLT registry, cystic fibrosis accounts for 53.8% of transplants in children aged between 6 and 11 years, and 71.5% in those aged between 12 and 17 years.1 It should be taken into consideration, however, that the ISHLT registry includes data from 1990, and that transplant in children under 11 and adolescents with cystic fibrosis is becoming increasingly less common. According to data from the Catalonian lung transplant registry (Spain), cystic fibrosis represents less than 10% of transplants performed in children under 11 years, but 85% of those carried out in adolescents aged between 12 and 17 years.17
When Should a Child Be Referred for Lung Transplantation?The decision to place a child on the waiting list for a lung transplant is not easy, as it is very difficult to predict survival. It is very important to make this decision properly, in order to provide a survival benefit. Some years ago in the USA, the benefit of lung transplantation in children with cystic fibrosis was questioned.18 This was possibly linked to too early inclusion on the waiting list in contrast to the improvement in survival of children with cystic fibrosis due to advances in medical treatment.11 With the adoption of a severity assessment system for prioritizing patients on the waiting list (Lung Allocation Score), a real benefit of transplantation in adults with cystic fibrosis been shown in the USA19; this scoring system is only applicable in children over 12 years of age. In other countries, a real benefit of lung transplantation has been demonstrated in children with cystic fibrosis and severe lung disease.20
The indications for referring a child with cystic fibrosis for evaluation for lung transplant can be summarized in: frequent hospital admissions for intravenous antibiotic treatment, major limitation for attending school or carrying out normal activities, rapid decline or marked fluctuations in lung function, including an FEV1 <30%, recurrent pneumothorax or hemoptysis, hypoxemia and hypercapnia.16,17 It is recommended that children be included if their life expectancy is less than 2 years despite receiving full medical therapy and they have poor quality of life that is likely to improve with transplantation.11 Using this approach, widely adopted in Europe, most of those who receive a transplant will benefit, although it has the disadvantage that some patients may die on the waiting list or even without being included on it if their prognosis is not properly assessed.11
In children with diffuse parenchymal lung diseases, the most important thing is to assess the presence of moderate or severe functional impairment, need for mechanical ventilation or very high oxygen supplementation, lack of response to treatment, and taking into account the natural course of the disease.15 With some diseases, such as surfactant protein B deficiency, the child cannot survive without a transplant, while others may have a variable response to treatment, as in surfactant protein C deficiency.16
The prognosis in pulmonary hypertension has improved considerably with the new treatments available, so the following criteria are now recommended: children in functional class IV: place on the transplant list and begin medical treatment; if they improve, remove them from the waiting list. Children in functional class III: administer medical treatment; if they do not improve or worsen, place them on the waiting list. Children with bilateral pulmonary vein stenosis or alveolar capillary dysplasia would have to be included on the transplant list at an early stage, as they do not respond to other treatments.13,16
The nature of these indications means that lung transplantation in mechanically ventilated children is indicated more often than in adults. Infants on mechanical ventilation or children on long-term mechanical ventilation may be considered candidates for lung transplant. It has been shown that mechanically ventilated infants who have received transplants have the same survival post-transplant as non-ventilated older children, and better than ventilated older children.21 Invasive mechanical ventilation in children with cystic fibrosis is a risk factor for higher post-transplant morbidity and mortality, unlike non-invasive ventilation.22–24
Table 3 summarizes the criteria for referring children to lung transplant teams.
Recommendations for Referring Children With Respiratory Disease to the Lung Transplant Team (Adapted From Sweet16).
| Disease | Indication for referral to transplant centre |
| Cystic fibrosis | Frequent hospital admissions for intravenous antibiotic treatment, major limitation for attending school or carrying out normal activities, rapid decline or marked fluctuations in lung function, including an FEV1 <30%, recurrent pneumothorax or hemoptysis, hypoxemia and hypercapnia |
| Idiopathic pulmonary hypertension | Functional class IV, right heart failure, hypoxia, recurrent syncope, no response to medical treatment |
| Pulmonary vein stenosis, alveolar capillary dysplasia | Must be referred at an early stage as they do not respond to treatment |
| Surfactant protein deficiencies | Patients with protein B deficiency in refractory respiratory failure should be referred at an early stage. Patients with ABCA-3 or protein C deficiency in the case of severe respiratory failure and no response to treatment. |
| Bronchopulmonary dysplasia | Given the possibility of progressive improvement of the disease, only patients with severe respiratory failure who do not improve over time should be referred. |
| Post-infectious bronchiolitis obliterans | Patients with major hypoxemia or requiring invasive mechanical ventilation who do not improve with medical treatment. Take into account the possibility of improvement over the first 2 years. |
| Other diffuse parenchymal lung diseases | Patients with major hypoxemia or requiring invasive mechanical ventilation who do not improve with medical treatment. |
There are several situations in which lung transplantation may not be advised, some absolute and others relative,17 while some are specific to each centre (Table 4).
Contraindications for Lung Transplantation.
| Absolute contraindications |
| Severe organic liver or renal dysfunction |
| Active extra-pulmonary infection (sepsis, tuberculosis) |
| Progressive neuromuscular disease |
| Active neoplasm in the previous 2–5 years (depending on the type of tumor) |
| Neurological dysfunction or severe chromosome abnormalities that impede good quality of life |
| Abnormal transpleural communications between the systemic and bronchial circulation |
| Relative contraindications |
| Burkholderia cepacia genomovar-3 infection |
| Severe scoliosis |
| Surgical pleurectomy or chemical pleurodesis |
| Severe tracheomalacia |
| Lung infection due to multiresistant pathogens |
| Difficulty in treatment compliance |
| Active collagen-vascular disease |
| Mechanical ventilation |
| Renal failure |
In patients with cystic fibrosis, Bulkholderia cepacia genomovar-3, Mycobacterium abscessus and multiresistant fungal infections (Schedosporium prolificans) constitute a contraindication in some centers.
Patients with cyanotic heart diseases in whom previous thoracotomies have been performed for palliative purposes have a very high risk of bleeding from chest wall collateral vessels, and should be assessed individually.
The Donor OrganOne of the current critical issues in pediatric lung transplantation, and pediatric transplants in general, is the fall in the number of young donors available, with the consequent increase in waiting list times. This is linked to the gradually decreasing number of donors as a result of road traffic accidents in the last 20 years, in parallel with the decrease in road fatalities, particularly after the introduction of new road traffic laws in Spain in 2005. While they represented 43% of donors in 1992, this figure fell to 5.7% of total donations in 2010.5
In our hospital, the median waiting time for children under 2 years old between the periods 1996–2005 and 2006–2011 increased moderately from 84 to 135 days, while for the 3–11 and 12–17 years age groups, it increased significantly, from 107 to 538 days and from 106.5 to 404.5 days, respectively.
In order to improve these figures, Pediatric Intensive Care Units must be extremely aware of the need to make the most of potential donors and to optimize their care, and strategies for using lungs from adult donors in children may be employed; it would also be advisable to explore the extension of non-heart-beating donor programs in pediatrics.8,25
Types of Transplant and Surgical TechniqueSequential double-lung transplant is typically carried out in children; this is compulsory in septic lung disease (cystic fibrosis) and is the procedure of choice in pulmonary hypertension. Single-lung transplantation is less indicated in children than in adults (only 1 out of 31 cases in the Spanish Lung Transplant Registry5). It can be performed in cases of non-septic parenchymal lung disease (pulmonary fibrosis, bronchiolitis obliterans, retransplantation), if the somatic growth is almost complete and the organ offered is of perfect size and quality. Heart-lung transplant is reserved for pulmonary vascular disease associated with uncorrectable congenital heart disease, and for cases in which there is left ventricular failure.
Unlike adults, most pediatric lung transplants are performed with CPB, as most children, due to their size, cannot tolerate single-lung ventilation. However, in adolescents and older children, it is sometimes possible to perform the transplant without CPB.2 Although it has been suggested that the use of this technique could be associated with a higher incidence of primary graft failure, in a large series comparing the frequency of primary graft dysfunction between transplants in adults and children, there were no differences in its incidence.26
Due to the difficulty in finding donors of a suitable size for children, there is greater flexibility in accepting size differences between the donor and recipient, and various techniques are performed to accommodate the lungs, including lobectomies (the middle lobe, lingula or lower lobes), non-anatomical resections, or using the lobes only. The upper or lower lobes can be used, but the upper lobes fit better in the thorax.27 Another strategy that has been used is to divide the left lung of an adult donor of suitable size, using the left lower lobe as a left lung and the left upper lobe as a right lung.27 There is experimental evidence in animal models that a “mature” lobe implanted in an immature recipient could grow by pneumocyte division.28
Living donor lobar lung transplantation requires 2 donors, extracting the lower lobe from each of them. This has significant morbidity for the donor and results similar to those of a deceased donor, so it is rarely used at present, except in Japan and Brazil and occasionally in some experienced centers in the USA.16
Special Features in Post-transplant Management and ComplicationsPost-transplant treatment and follow-up is similar to that in adult patients, although there are some major differences.
ImmunosuppressionMost pediatric centers use triple immunosuppressant therapy with tacrolimus, mycophenolate and corticosteroids.2 Children under 5 years of age have increased clearance of calcineurin inhibitors, which means that higher doses are required in younger recipients; infants may require 2–4 times the adult dose.29 This higher clearance together with more rapid absorption means that there is greater fluctuation in levels, and that more frequent administration (every 8h) may be required in small children. Cystic fibrosis patients require a 40%–50% higher dose of tacrolimus due to poorer absorption and higher clearance. A good alternative may be sublingual tacrolimus, which achieves more stable levels with lower doses.30
MonitoringIt is impossible to apply standard chronic graft dysfunction diagnostic criteria in small children. Spirometry can only be performed after children reach 3–4 years of age, and more reliably from 6 years old upwards. Although there are specific lung function tests in infants, such as forced thoracic compression after prior insufflation, diagnostic criteria using these techniques have not been validated.
Biopsy forceps that can be used with 2.8 and 3.6mm pediatric bronchoscopes often do not allow adequate samples to be obtained for the diagnosis of acute rejection or assessment of the airways. The alternative may be to use standard forceps through a rigid bronchoscope, or on rare occasions, to perform an open lung biopsy.
Thus, in small children, it is essential to rely on clinical symptoms, SaO2 values and chest computed tomography with inspiratory and expiratory slices.
ComplicationsThe same complications can occur in children as in adult patients following lung transplantation, with some that are specific to children.
Despite having smaller anatomical structures, vascular anastomotic and airway complications have a frequency in all pediatric age groups similar to that in adults.31
Small children are more prone to viral respiratory infections, most of which are well tolerated. Although an impact on mortality and the development of chronic graft dysfunction has not been reported in any pediatric series,32 community respiratory virus infection, such as respiratory syncytial virus (RSV), adenovirus, metapneumovirus, etc. may lead to graft failure or death of the patient. Most pediatric centers administer prophylaxis against RSV using specific antibodies (palivizumab).33
Children who have undergone transplant have an increased risk of neoplasia, the most common of which is lymphoproliferative syndrome related with Epstein-Barr virus infection, with an incidence ranging from 4% one year post-transplant to 15% after 5 years.34
Prognosis and SurvivalLung transplant recipients experience a major improvement in quality of life, and 88% of children do not have any limitations in their activity 3 years after the transplant.1
According to data from the ISHLT registry, survival 5 years post-transplant is 54% and after 10 years is around 30%, with survival in the 0–1 year and 1–11 year age groups better than that in the 12–17 year age group (mean survival of 6.4 years for infants, 6–6.7 years for small children, and 4.6 years for adolescents).1 One of the causes of the lower survival rate in adolescents is likely poorer treatment compliance in this age group.35 Moreover, infants and preschool children appear to have better immune tolerance to the allograft and a lower incidence of acute rejection and chronic graft dysfunction than older children and adults.36
Survival data in Spain are similar to those of the ISHLT registry. In our centre, survival was 55% at 5 years and 47% at 7 years,12 and in the Spanish transplant registry,6 the one-year survival was 90.3% and 3-year survival was 66.9%.
In cases in which the transplant fails, the only alternative is retransplantation, which may be limited by donor availability. In a recent study in the United States, it was observed that the survival after retransplantation was similar to that of the primary transplant if performed after the first year of transplant in unventilated cases, but it was poorer if performed within the first year of transplant or in ventilated patients.37
The advances made in recent years in surgical techniques and immunosuppression mean that lung transplant in children is a reality. Efforts are needed to increase the number of donors available, and to further improve long-term results.
Conflict of InterestThe authors declare that they do not have any conflict of interests.
Please cite this article as: Moreno Galdó A, Solé Montserrat J, Roman Broto A. Trasplante pulmonar en niños. Aspectos específicos. Arch Bronconeumol. 2013;49:523–528.