Adverse respiratory events (AREs) are leading causes of postoperative morbidity and mortality. This study investigated the incidence and determinants of postoperative ARE.
MethodsThis observational prospective study was conducted in a post-anesthesia care unit (PACU). A total of 340 adult subjects were admitted consecutively, and AREs were measured after elective surgery. Population demographics, perioperative parameters, ARE occurrence, and length of stay in the postoperative PACU and in hospital were recorded. Data were analyzed descriptively using the Mann–Whitney U-test and the Chi-square or Fisher's exact test. Multivariate analyses were carried out using logistic binary regression, and the odds ratio (OR) and 95% confidence interval (CI) were calculated.
ResultsPostoperative AREs occurred in 67 subjects (19.7%). AREs were more frequent after high-risk procedures (42% vs 24%; P=.003), in patients undergoing major surgery (37% vs 25%; P=.041), those receiving general anesthesia (85% vs 67%; P=.004), and in patients administered intraoperative muscle relaxants (79% vs 55%; P<.001) and neostigmine (69% vs 49%; P=.002). Hypoactive emergence (13% vs 5%; P=.015) and residual neuromuscular blockade (46% vs 11%; P<.001) were more frequent in subjects with postoperative ARE. On multivariate analyses, residual neuromuscular blockade was an independent risk factor for ARE in the PACU (OR 6.4; CI 3.0–13.4; P<.001).
ConclusionsARE is an important and common postoperative complication. Residual neuromuscular blockade was an independent risk factor for ARE in the PACU.
Los acontecimientos adversos respiratorios (AAR) constituyen causas importantes de morbimortalidad postoperatoria. En el presente estudio se investigó la incidencia y los factores determinantes de los AAR postoperatorios.
MétodosEste estudio prospectivo observacional se llevó a cabo en una unidad de cuidados postanestésicos (UCPA). Se estudió a un total de 340 pacientes adultos ingresados de forma consecutiva, y se evaluaron los AAR después de intervenciones de cirugía programada. Se registraron las características demográficas de la población, los parámetros perioperatorios, la frecuencia de AAR y la duración de la estancia postoperatoria en la UCPA y en el hospital. Los datos se analizaron mediante estadística descriptiva, con la prueba de U de Mann-Whitney y con la prueba de χ2 o la prueba exacta de Fisher. Se realizaron análisis multivariantes con una regresión binaria logística, y se calculó la odds ratio (OR) y su intervalo de confianza (IC) del 95%.
ResultadosSe produjeron AAR postoperatorios en 67 pacientes (19,7%). Los AAR fueron más frecuentes después de intervenciones quirúrgicas de riesgo alto (42% frente a 24%; p=0,003), en los pacientes a los que se practicaron operaciones de cirugía mayor (37% frente a 25%, p=0,041), en los que fueron intervenidos bajo anestesia general (85% frente a 67%, p=0,004) y en los pacientes a los que se administraron relajantes musculares intraoperatorios (79% frente a 55%; p<0,001) y neostigmina (69% frente a 49%, p=0,002). Los pacientes con AAR postoperatorios experimentaron con mayor frecuencia una emergencia hipoactiva (13% frente a 5%, p=0,015) y un bloqueo neuromuscular residual (46% frente a 11%, p<0,001). En los análisis multivariantes, el bloqueo neuromuscular residual fue un factor de riesgo independiente para la aparición de los AAR en la UCPA (OR 6,4; IC 3,0-13,4; p<0,001).
ConclusionesLos AAR constituyen una complicación postoperatoria importante y frecuente. El bloqueo neuromuscular residual fue un factor de riesgo independiente para la aparición de AAR en la UCPA.
Adverse respiratory events (AREs) are still one of the most important causes of postoperative morbidity and mortality.1 The reported incidence of postoperative respiratory complications ranges from 5% to 80%, depending on the patient population and the diagnostic criteria used to define complications.2 Respiratory episodes that occur immediately after a surgical intervention are common, and ARE rates of 1.3%–6.9% have been reported after admission to a post-anesthesia care unit (PACU).3,4
Respiratory problems are common in the PACU.5 The vast majority are attributable to airway obstruction, hypoventilation or hypoxemia. Hypoxemia, irrespective of its etiology, is generally the final stage in a process leading to severe morbidity and mortality, and may be a result of hypoventilation or compromised alveolar oxygen exchange causing a rise in the alveolar-arterial gradient. Patients with significant comorbidities, particularly neuromuscular, pulmonary or cardiac dysfunction, have a higher risk of respiratory compromise, but all patients are at risk of developing postoperative hypoxemia, which occurs in 30%–50% of abdominal surgery and in up to 8%–10% of patients who require intubation and mechanical ventilation.6
In the immediate postoperative period, the most common causes of hypoventilation are airway obstruction, anesthetics, residual neuromuscular blockade (RNMB) and breathing difficulty due to poor control of incision wound pain.4,5,7–11 Postoperative oxygen exchange deficit may occur as a result of intrapulmonary shunt, lung edema and embolism.12 Pre-existing medical disorders, such as obstructive sleep apnea, may also make the patient more susceptible to airway obstruction in the PACU.13
The most common cause of airway obstruction in the PACU is pharyngeal floppiness caused by pharyngeal muscle weakness.11 Other causes include laryngeal spasm, airway edema, hematoma or presence of a foreign body.
Muscle weakness is a result of loss of pharyngeal motor tone caused by RNMB, the enduring effect of anesthetics, or narcotic drugs.10,14,15
The diaphragm recovers more quickly from the effect of muscle relaxants than the pharynx. Residual pharyngeal muscle paralysis causes the base of the tongue and the posterior oropharyngeal tissues to bunch together, producing supraglottal obstruction.16,17
The aim of this study was to determine the incidence and predictive factors of postoperative ARE in the PACU.
MethodsDesign, Setting and ParticipantsThis prospective, observational study was performed in the Centro Hospitalar São João (Porto, Portugal), a tertiary-level hospital with 1124 beds, over a 3-week period between May 9 and May 27, 2011. Patients were admitted to the 13-bed PACU, where they were monitored and treated. The study was approved by the Ethics Committee of the Centro Hospitalar São João. Informed consent was obtained during the preoperative consultation. ARE incidence during hospitalization in the PACU was recorded in consecutive, Portuguese-speaking adult patients, admitted to the PACU after elective surgery. Patients undergoing neurological, thoracic or pulmonary surgery are not transferred to the PACU but are instead admitted to intensive and intermediary care units.
Inclusion criteria were ability to give written informed consent and spontaneous ventilation on admission.
Patients who received programmed mechanical ventilation after surgery and needed to be admitted for an overnight stay in the intensive care unit (ICU) were not transferred to the PACU and were excluded from the study. Other criteria for exclusion were patient's refusal to participate, inability to give informed consent, Mini-Mental State Examination (MMSE) score <25, known neuromuscular disease, urgent or very urgent surgery, neurosurgery and interventions requiring therapeutic hypothermia.
Interventions and ParticipantsBefore surgery, participants were interviewed briefly and consent was obtained, the MMSE scale18 was administered, and disease history was recorded. During the interview, patients completed the STOP-BANG (snoring, daytime tiredness, observed apnea, high blood pressure, body mass index, age, neck girth and gender) questionnaire.19 This questionnaire was validated for a surgical population by Chung et al.19 and is based on 8 easily answered questions with yes/no answers (score: 1/0), scored from 0 to 8. A score of ≥3 is highly sensitive for detecting obstructive sleep apnea. Data were also collected on body mass index (BMI), age, neck girth and gender (BANG). Patients with a STOP-BANG of 3 or higher were classified as high risk for obstructive sleep apnea (OSA).
A standardized form recording age, weight, height, BMI and physical status according to the American Society of Anesthesiologists (ASA-PS) was completed for each patient. The Revised Cardiac Risk Index (RCRI)20 was calculated, including high risk surgery (i.e., intraperitoneal, intrathoracic, or suprainguinal vascular surgery) and clinical risk factors (previous ischemic heart disease, compensated heart failure, heart failure, cerebrovascular disease, diabetes mellitus and kidney failure).
The surgical intervention was classified as major (body cavities or main blood vessels exposed to room temperature), medium (less exposure of body cavities) or minor (superficial surgery). Major surgery was defined as procedures needing 2 or more days of hospitalization.
AnesthesiaThe treating anesthesiologist was unaware of the patient's participation in the study and was not informed that the research was being conducted. Anesthesia was administered and supervised at the discretion of the anesthesiologist, following departmental minimum standards. Neuromuscular blocking agents (NMBA) were administered for tracheal intubation and additionally if required. Neuromuscular monitoring is not defined by a written policy in our center, so this was carried out at the discretion of the anesthesiologist.
Patients were usually extubated in the surgical area and then transferred to the PACU. Criteria for extubation were unassisted head lift or hand grip for at least 5s, ability to follow simple commands, stable ventilatory pattern with acceptable oxygen saturation (SpO2>95%) and contractile response to train-of-four (TOF) stimulation greater than 0.80. All patients received 100% oxygen via a mask after tracheal extubation. The anesthesiologist decided if oxygen had to be administered or not during transfer and admission to the PACU.
On arrival in the PACU, oxygen was administered to all patients via a nasal cannula or mask. RNMB was defined as TOF <0.9 quantified on admission to the PACU with an acceleromyography at the adductor pollicis of the thumb (TOF-Watch®).
Other intraoperative parameters recorded were type of anesthesia, surgical intervention, duration of anesthesia, duration of procedure, intraoperative fluids, use of NMBAs and use of suxamethonium.
Post-Operative Data and Adverse Respiratory EventsAll post-surgery AREs were recorded in the case report form, according to the criteria described by Murphy et al.21 as follows:
- 1.
Upper airway obstruction requiring intervention (jaw thrust, oral airway or nasal airway);
- 2.
Mild-moderate hypoxemia (SpO2=93%–90%) on 3L nasal cannula oxygen that was not improved after active interventions (increasing O2 flows to >3L/min, application of high-flow face mask O2, verbal requests to breathe deeply, tactile stimulation);
- 3.
Severe hypoxemia (SpO2<90%) on 3L nasal cannula O2 that was not improved after active interventions (increasing O2 flows to >3L/min, application of high-flow facemask O2, verbal requests to breathe deeply, tactile stimulation);
- 4.
Signs of respiratory distress or impending ventilatory failure (respiratory rate >20 breaths per minute, accessory muscle use, tracheal tug);
- 5.
Inability to breathe deeply when requested to by the PACU nurse;
- 6.
Patient complaining of symptoms of respiratory or upper airway muscle weakness (difficulty breathing, swallowing or speaking);
- 7.
Patient requiring reintubation in the PACU;
- 8.
Clinical evidence or suspicion of pulmonary aspiration after tracheal extubation (gastric contents observed in the oropharynx and hypoxemia).
A team of qualified doctors evaluated patients’ emergence from anesthesia. Patients were monitored 10min after arrival in the PACU. Emergence delirium and hypoactive emergence and severity were diagnosed according to the Richmond Agitation and Sedation Scale (RASS).22,23 Emergence delirium was defined as RASS≥+1 and hypoactive emergence as RASS≤–2.
The Nursing Delirium Screening Scale (Nu-DESC)24 was used to detect delirium at the time of discharge from the PACU; patients with a score of 2 or more after at least 1 evaluation were considered positive for delirium. The team of investigators evaluated delirium when the patient was formally declared ready for discharge and transfer to a general ward. PACU stay and hospital stay were also recorded.
Statistical AnalysisStudy data were summarized in a descriptive analysis of the variables. Univariate analyses were performed using the Mann–Whitney U test for comparing continuous variables and the χ2 test and Fisher's exact test were used for comparing proportions.
All variables were forced into multiple binary logistic regression models, in order to identify independent predictive factors for ARE. All covariates with a P value <.05 in the univariate analysis were also included in this model. Data were analyzed using SPSS for Windows version 19.0 (SPSS, Chicago, IL, USA).
ResultsA total of 357 patients were admitted consecutively to the PACU during the study period and gave their consent to participate; of these, 340 participants were evaluated.
Seventeen patients were excluded: 7 were admitted to the surgical ICU; 3 could not give informed consent or had an MMSE score of <25; 3 did not undergo surgery; 1 was under 18 years of age; 1 did not speak Portuguese; 1 refused to participate; 1 underwent a neurosurgical procedure.
Median age was 57 years and median BMI was 26kg/m2. Demographic characteristics of the population, surgical details, anesthetic management and PACU data are summarized in Tables 1–3.
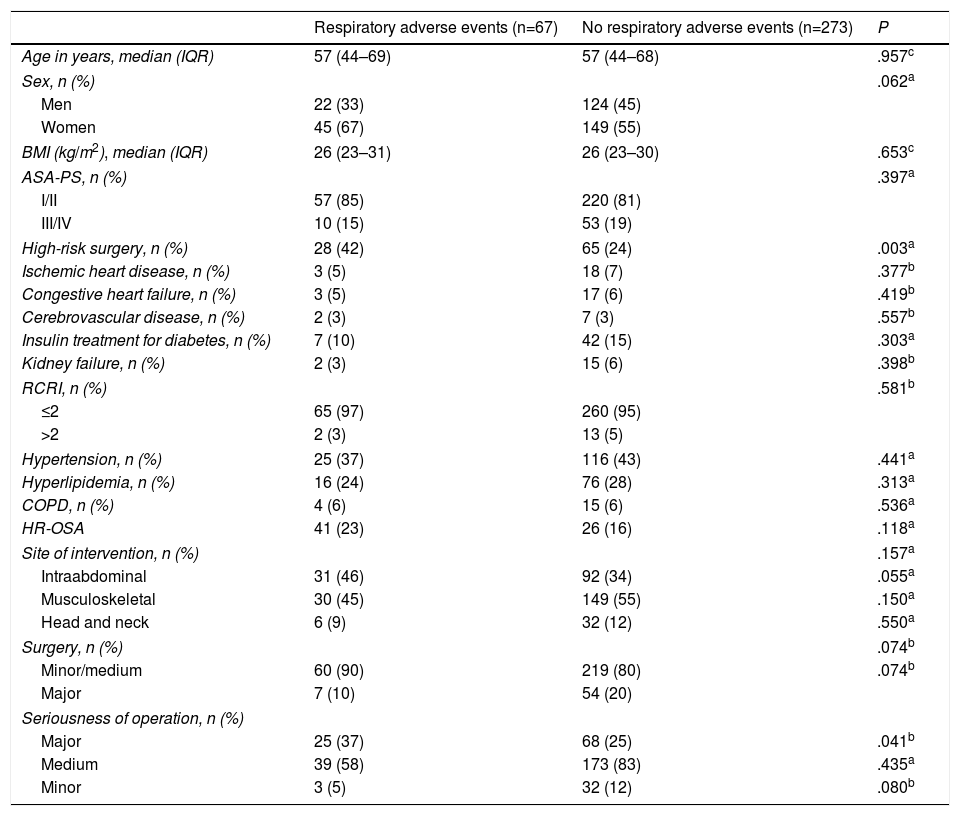
Patient Characteristics and Adverse Respiratory Events in the Post-Anesthesia Care Unit.
| Respiratory adverse events (n=67) | No respiratory adverse events (n=273) | P | |
|---|---|---|---|
| Age in years, median (IQR) | 57 (44–69) | 57 (44–68) | .957c |
| Sex, n (%) | .062a | ||
| Men | 22 (33) | 124 (45) | |
| Women | 45 (67) | 149 (55) | |
| BMI (kg/m2), median (IQR) | 26 (23–31) | 26 (23–30) | .653c |
| ASA-PS, n (%) | .397a | ||
| I/II | 57 (85) | 220 (81) | |
| III/IV | 10 (15) | 53 (19) | |
| High-risk surgery, n (%) | 28 (42) | 65 (24) | .003a |
| Ischemic heart disease, n (%) | 3 (5) | 18 (7) | .377b |
| Congestive heart failure, n (%) | 3 (5) | 17 (6) | .419b |
| Cerebrovascular disease, n (%) | 2 (3) | 7 (3) | .557b |
| Insulin treatment for diabetes, n (%) | 7 (10) | 42 (15) | .303a |
| Kidney failure, n (%) | 2 (3) | 15 (6) | .398b |
| RCRI, n (%) | .581b | ||
| ≤2 | 65 (97) | 260 (95) | |
| >2 | 2 (3) | 13 (5) | |
| Hypertension, n (%) | 25 (37) | 116 (43) | .441a |
| Hyperlipidemia, n (%) | 16 (24) | 76 (28) | .313a |
| COPD, n (%) | 4 (6) | 15 (6) | .536a |
| HR-OSA | 41 (23) | 26 (16) | .118a |
| Site of intervention, n (%) | .157a | ||
| Intraabdominal | 31 (46) | 92 (34) | .055a |
| Musculoskeletal | 30 (45) | 149 (55) | .150a |
| Head and neck | 6 (9) | 32 (12) | .550a |
| Surgery, n (%) | .074b | ||
| Minor/medium | 60 (90) | 219 (80) | .074b |
| Major | 7 (10) | 54 (20) | |
| Seriousness of operation, n (%) | |||
| Major | 25 (37) | 68 (25) | .041b |
| Medium | 39 (58) | 173 (83) | .435a |
| Minor | 3 (5) | 32 (12) | .080b |
ASA-PS, American Society of Anesthesiologists-Performance Status; COPD, chronic obstructive pulmonary disease; HR-OSA, high-risk of obstructive sleep apnea; BMI, body mass index; RCRI, Revised Cardiac Risk Index; IQR, interquartile range.
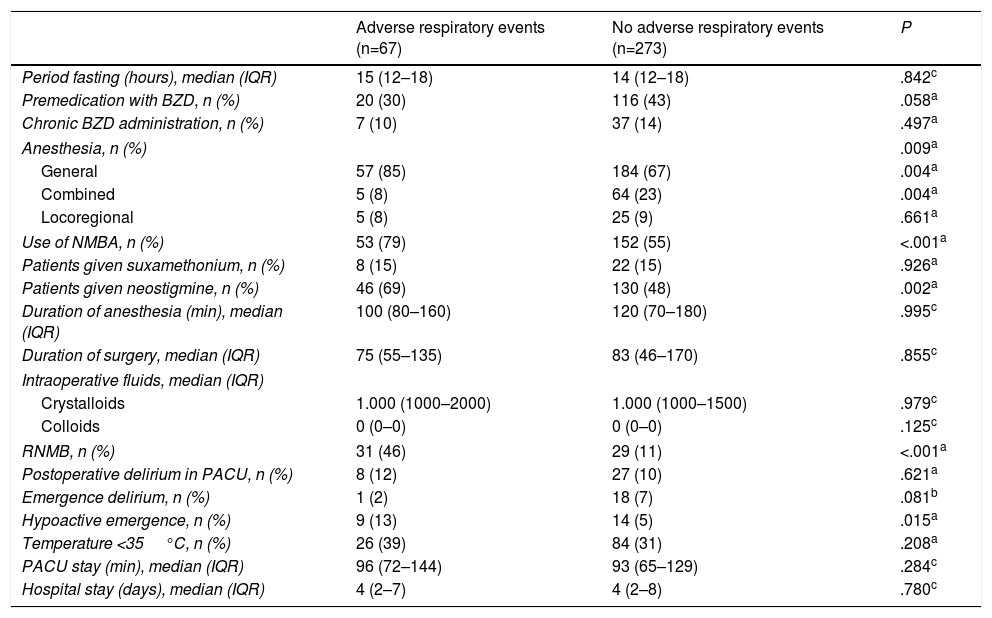
Perioperative Characteristics and Postoperative Adverse Respiratory Events in the Post-Anesthesia Care Unit.
| Adverse respiratory events (n=67) | No adverse respiratory events (n=273) | P | |
|---|---|---|---|
| Period fasting (hours), median (IQR) | 15 (12–18) | 14 (12–18) | .842c |
| Premedication with BZD, n (%) | 20 (30) | 116 (43) | .058a |
| Chronic BZD administration, n (%) | 7 (10) | 37 (14) | .497a |
| Anesthesia, n (%) | .009a | ||
| General | 57 (85) | 184 (67) | .004a |
| Combined | 5 (8) | 64 (23) | .004a |
| Locoregional | 5 (8) | 25 (9) | .661a |
| Use of NMBA, n (%) | 53 (79) | 152 (55) | <.001a |
| Patients given suxamethonium, n (%) | 8 (15) | 22 (15) | .926a |
| Patients given neostigmine, n (%) | 46 (69) | 130 (48) | .002a |
| Duration of anesthesia (min), median (IQR) | 100 (80–160) | 120 (70–180) | .995c |
| Duration of surgery, median (IQR) | 75 (55–135) | 83 (46–170) | .855c |
| Intraoperative fluids, median (IQR) | |||
| Crystalloids | 1.000 (1000–2000) | 1.000 (1000–1500) | .979c |
| Colloids | 0 (0–0) | 0 (0–0) | .125c |
| RNMB, n (%) | 31 (46) | 29 (11) | <.001a |
| Postoperative delirium in PACU, n (%) | 8 (12) | 27 (10) | .621a |
| Emergence delirium, n (%) | 1 (2) | 18 (7) | .081b |
| Hypoactive emergence, n (%) | 9 (13) | 14 (5) | .015a |
| Temperature <35°C, n (%) | 26 (39) | 84 (31) | .208a |
| PACU stay (min), median (IQR) | 96 (72–144) | 93 (65–129) | .284c |
| Hospital stay (days), median (IQR) | 4 (2–7) | 4 (2–8) | .780c |
NMBA, neuromuscular blocking agents; RNMB, residual neuromuscular blockage; BZD, benzodiazepines; IQR, interquartile range; PACU, post-anesthesia care unit.
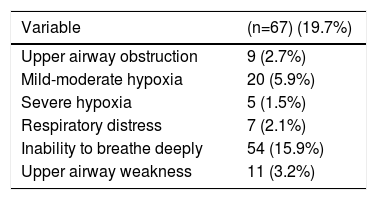
A total of 67 patients (19.7%) had ARE (Table 4). AREs most commonly observed were the following: inability to breathe deeply (15.9%), mild-moderate hypoxemia (5.9%) and upper airway weakness (3.2%). Other AREs recorded were upper airway obstruction (2.7%), signs of respiratory distress or impending ventilatory failure (2.1%) and severe hypoxemia (1.5%). None of the patients required reintubation and none had clinical evidence or suspicion of pulmonary aspiratory during their PACU stay. Multiple AREs were observed in 21 patients (6.2%).
Incidence of Acute Respiratory Episodes in the Post-Anesthesia Care Unit (n=340).
| Variable | (n=67) (19.7%) |
|---|---|
| Upper airway obstruction | 9 (2.7%) |
| Mild-moderate hypoxia | 20 (5.9%) |
| Severe hypoxia | 5 (1.5%) |
| Respiratory distress | 7 (2.1%) |
| Inability to breathe deeply | 54 (15.9%) |
| Upper airway weakness | 11 (3.2%) |
The most common AREs occurred in patients who had undergone high-risk procedures (42% vs 24%, P=.003) and major surgery (37% vs 25%, P=.041).
AREs were more common in patients who were given general anesthesia (85% vs 67%, P=.004) and less common in patients given combined anesthesia (8% vs 23%, P=.004).
Five patients who received locoregional anesthesia developed inability to breathe deeply.
Neuromuscular relaxants were used in 66% of the patients: AREs occurred more commonly in these patients (79% vs 55%, P<.001). AREs were also more common in patients who received neostigmine (69% vs 48%, P=.002).
Neither duration of anesthesia nor duration of intervention affected the incidence of ARE.
AREs were more common in patients with RNMB (46% vs 11%, P<.001) and in patients with a diagnosis of hypoactive emergence (13% vs 5%, P=.015).
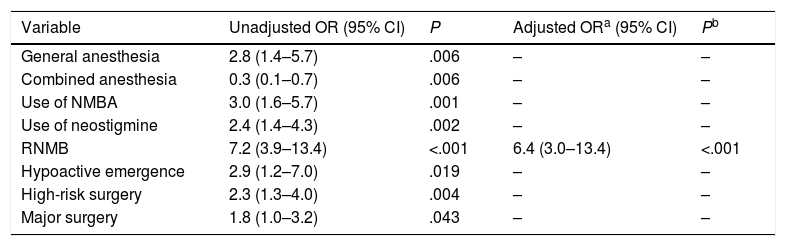
Multivariate analyses (Table 5) performed after adjustment for univariate predictive factors (general anesthesia, combined anesthesia, use of neuromuscular relaxant and neostigmine, hypoactive emergence, and major and high-risk surgery), revealed RNMB as an independent predictive risk factor for ARE.
Multivariate Regression Analysis for Predictive Factors for Acute Respiratory Episodes.
| Variable | Unadjusted OR (95% CI) | P | Adjusted ORa (95% CI) | Pb |
|---|---|---|---|---|
| General anesthesia | 2.8 (1.4–5.7) | .006 | – | – |
| Combined anesthesia | 0.3 (0.1–0.7) | .006 | – | – |
| Use of NMBA | 3.0 (1.6–5.7) | .001 | – | – |
| Use of neostigmine | 2.4 (1.4–4.3) | .002 | – | – |
| RNMB | 7.2 (3.9–13.4) | <.001 | 6.4 (3.0–13.4) | <.001 |
| Hypoactive emergence | 2.9 (1.2–7.0) | .019 | – | – |
| High-risk surgery | 2.3 (1.3–4.0) | .004 | – | – |
| Major surgery | 1.8 (1.0–3.2) | .043 | – | – |
NMBA, neuromuscular blocking agents; RNMB, residual neuromuscular blockage; CI, confidence interval; OR, odds ratio.
All variables with statistical significance in the univariate analysis were forced into the logistic regression model.
A total of 20% of the patients had at least 1 ARE during their PACU stay.
The reported incidence of AREs recorded in the PACU varies widely. Observational studies have described rates of 1.3%–34%,4,25,26 depending on the definition of ARE, the duration of monitoring and the patient population. Murphy et al.26 found a rate of mild hypoxia of 22%, similar to the findings of this study, but the same author described a much lower rate in another study.
Patients presenting ARE in the PACU are more likely to have undergone high-risk surgical interventions, defined according to the RCRI.20 This index was initially designed to predict major cardiac episodes, but it is also applicable to other post-operative complications.27
AREs were also more common after major surgery; these patients may have received different anesthetic management, closer monitoring, and probably a longer surgical intervention. They were also more likely to have a poor preoperative status, which may also have affected the incidence of ARE.
Abdominal, vascular and thoracic surgeries were also defined as the major procedures by Warner,28 who found that postoperative hypoxemia and acute respiratory failure mainly occurred after abdominal or thoracic surgery.
The overall incidence of RNMB in the study population was 18%. Patients with ARE had a higher rate of RNMB than patients without ARE.
It is interesting to note that more patients with ARE had received neostigmine. Most anesthesiologists systematically administered a muscle relaxant reversal agent, but some may have used subjective criteria.
Many centers in Europe have stated that they do not use reversal agents.29–31 However, in view of the current evidence, this practice is not recommended in the standard management of these patients.32 In the hospital where this study was performed, reversal agents are generally administered to all patients as a standard procedure. This may explain the higher incidence of ARE in patients who received neostigmine.
Delayed PACU discharge due to ARE is difficult to evaluate, and depends on individual factors in each center, such as staffing resources, PACU size and availability of beds. A recent study suggested that AREs caused a delay in discharge from the recovery room,33 but we found no differences between the 2 study groups, both of which had a short mean PACU stay; the similarity in PACU stay in patients with or without ARE may be explained by the availability of intermediate surgical care units.
AREs were more common after general anesthesia: this was not unexpected and has been documented in previous studies.4 However, the protective effect of general anesthesia compared to combined anesthesia in our study was surprising. It may be that administration of NMBA is rarer in combined anesthesia and analgesia is more effective if not combined with narcotics.
No relation was found between duration of the surgical procedure and AREs; however, Canet34 suggested that duration of both anesthesia and procedure may be related with postoperative pulmonary complications after PACU discharge. These episodes may have been missed, since the period of observation in our study was the duration of PACU stay.
Hypoactive emergence, defined as a RASS score of ≤–2, was more common in patients with ARE, suggesting that these patients were more heavily sedated on PACU admission.35
Compared to the findings of Radtke et al.,35 our population had a higher rate of emergence problems (12%), mainly due to hypoactive emergence in 6.8% of patients.
Abdominal surgery is a risk factor that has been associated with hypoactive emergence,36 and may have contributed to the association observed between high-risk surgery and the incidence of RNMB. Abdominal surgery can also be related with the higher rate of hypoactive delirium in RNMB patients. Another possibility is that the relationship between ARE and hypoactive emergence may reflect the level of sedation of the patient, which increases the chance of hypoxemia and atelectasis in a non-ventilated patient.
The most common ARE during PACU stay was inability to breathe deeply, recorded in 16% (54 of 340) of all patients and 81% (54 of 67) of the patients with ARE. This high rate may be due to the association of RNMB and a hypoactive status. Mild-moderate hypoxemia was the second most common ARE, appearing in 6% (20 of 340) of all patients and in 29.9% (20 of 67) of those with ARE. An association between RNMB and postoperative hypoxemia has been described in other studies, but this study was not designed to identify the underlying mechanism of neuromuscular block (NMB)-induced hypoxemia. Murphy et al.21 affirmed that deterioration of the ventilatory response to hypoxia and respiratory muscle weakness are important causes of postoperative hypoxemia, but these causes cannot be evaluated in an ordinary clinical examination. Upper airway obstruction, another common cause of ARE, occurred at a rate of 3% (9 of 340) in the study population, accounting for 13% of all AREs. Inhaled anesthetics and narcotics can be considered as relevant factors, but RNMB may also play an important role.
Our study has several limitations that must be mentioned. First, the sample is small and the study was conducted in a single center, making it difficult to generalize our results beyond our hospital. Second, it was a prospective, observational study. No interventions were made in anesthetic practice before, during, or after surgery, and all treatments were administered according to the criteria of the clinician who was in charge of the PACU that day.
Furthermore, the definition of ARE contains several subjective criteria that may have affected diagnosis. Finally, interoperative NMB status was not monitored, and this may have affected postoperative respiratory function. This would also limit the interpretation of the influence of neostigmine administration and the duration of NMB. Although intraoperative acceleromyography has been shown to reduce the risk of ARE in the PACU,26 this benefit is less pronounced when reversal agents are systematically administered.37
To conclude, AREs are a common occurrence in the PACU, and are more common after high-risk surgical interventions and major surgery. Postoperative RNMB, hypoactive emergence and the administration of neostigmine and interoperative muscle relaxants were predictive factors for developing ARE. RNMB was an independent risk factor for postoperative AREs in the PACU.
AuthorshipFernando Abelha and Alice Santos contributed to conception and design. Fernando Abelha contributed to analysis and interpretation. Alice Santos and Daniela Xará contributed to preparation of the significant intellectual content of the paper.
Conflict of InterestsThe authors have no conflict of interests.
Please cite this article as: Xará D, Santos A, Abelha F. Acontecimientos adversos respiratorios en la unidad de cuidados postanestésicos. Arch Bronconeumol. 2015;51:69–75.