Obesity is associated with 2 closely related respiratory diseases: obesity hypoventilation syndrome (OHS) and obstructive sleep apnea–hypopnea syndrome (OSAHS). It has been shown that noninvasive ventilation during sleep produces clinical and functional improvement in these patients. The long-term survival rate with this treatment, and the difference in clinical progress in OHS patients with and without OSAHS are analyzed.
MethodologyLongitudinal, observational study with a cohort of patients diagnosed with OHS, included in a home ventilation program over a period of 12 years, divided into 2 groups: pure OHS and OSAHS-associated OHS. Bi-level positive airway pressure ventilation was administered. During the follow-up period, symptoms, exacerbations and hospitalizations, blood gas tests and pulmonary function tests, and survival rates were monitored and compared.
ResultsEighty-three patients were eligible for analysis, 60 women (72.3%) and 23 men (27.7%), with a mean survival time of 8.47 years. Fifty patients (60.2%) were included in the group without OSAHS (OHS) and 33 (39.8%) in the OSAHS-associated OHS group (OHS–OSAHS). PaCO2 in the OHS group was significantly higher than in the OHS–OSAHS group (P<.01). OHS patients also had a higher hospitalization rate (P<.05). There was a significant improvement in both groups in FEV1 and FVC, and no differences between groups in PaCO2 and PaO2 values. There were no differences in mortality between the 2 groups, but low FVC values were predictive of mortality.
ConclusionsThe use of mechanical ventilation in patients with OHS, with or without OSAHS, is an effective treatment for the correction of blood gases and functional alterations and can achieve prolonged survival rates.
Desde el punto de vista respiratorio, la obesidad se asocia con 2 enfermedades muy relacionadas: el síndrome de obesidad-hipoventilación (SOH) y el síndrome de apnea-hipopnea del sueño (SAHS). Se ha demostrado que el tratamiento con ventilación mecánica no invasiva durante el sueño produce una mejoría clínica y funcional en estos pacientes. Analizamos a largo plazo la supervivencia con este tratamiento, y la diferencia en la evolución entre pacientes con SOH con y sin SAHS asociado.
MetodologíaEstudio longitudinal, observacional, de una cohorte de pacientes diagnosticados de SOH e incluidos en un programa de ventilación domiciliaria a lo largo de 12 años, distribuidos en 2 grupos: SOH puro y SOH asociado a SAHS. La ventilación se llevó a cabo con ventiladores de presión positiva continua binivel. Durante el tiempo de seguimiento se monitorizó y comparó su situación clínica (síntomas, exacerbaciones e ingresos), gasométrica y funcional, así como su supervivencia.
ResultadosOchenta y tres pacientes fueron válidos para el análisis, 60 mujeres (72,3%) y 23 hombres (27,7%), con una media de supervivencia de 8,47 años. Cincuenta pacientes (60,2%) fueron incluidos en el grupo sin SAHS (SOH) y 33 (39,8%) en el grupo con SAHS (SOH-SAHS). La PaCO2 del grupo SOH era significativamente mayor que la del grupo SOH-SAHS (p<0,01), y también presentaban más hospitalizaciones (p<0,05). Existió una mejoría significativa en ambos grupos en FEV1 y FVC, y en los valores de PaCO2 y PaO2, sin diferencias entre los grupos. Mientras que no se apreciaron diferencias en la supervivencia relacionadas con el subgrupo diagnóstico, valores bajos de FVC sí constituían un factor predictivo de mortalidad.
ConclusionesEl uso de ventilación domiciliaria en pacientes con SOH con o sin SAHS es un tratamiento eficaz que corrige las alteraciones gasométricas y funcionales y permite alcanzar tiempos prolongados de supervivencia.
Obesity is the most prevalent metabolic disease in the Western world. It is associated with high rates of morbidity and mortality, and has been recognized by the World Health Organization as a serious health, social and economic problem.1 It produces significant changes in the physiology of the respiratory system that can give rise to a wide spectrum of clinical manifestations, ranging from secondary dyspnea and restrictive ventilatory defect to hypercapnic respiratory failure characteristic of obesity-hypoventilation syndrome (OHS).2–4
OHS was first described in 19565 and is currently defined as a condition characterized by obesity, hypercapnia and respiratory changes during sleep, in the absence of any other disease that could explain respiratory failure.6 Despite the passage of time, the pathogenesis of this entity has still not been fully explained, although it seems to have a multifactorial origin in which altered ventilatory mechanics, muscle dysfunction secondary to obesity and ventilation control play an important role.7,8 Many authors consider it as simply a progressive form of the obstructive sleep apnea–hypopnea syndrome (OSAHS) in some obese subjects.9 However, although respiratory disorders during sleep are a defining condition for OHS, they are not always associated with OSAHS10 and it remains unknown which subgroup will progress the best. The prevalence of OHS in the general obese population is not precisely defined, but in obese patients with OSAHS it ranges between 10% and 20%.11,12 Studies in hospitalized obese patients have shown greater prevalence, also revealing increased mortality rates in this group.13
Since its introduction for the treatment of OSAHS, continuous positive airway pressure (CPAP) has also become a treatment for OHS, with non-invasive mechanical ventilation (NIV) being reserved for cases that do not respond adequately to positive pressure.14–16 Numerous groups initially rejected the use of NIV as standard treatment for OHS, but it was shown that in this group of patients similar results were obtained to those observed in patients with respiratory failure due to chest wall dysfunction,17 both in acute situations or in long-term home use.18 NIV to treat night-time OHS produces clinical and functional improvement with favorable changes in blood gases, polyglobulia, respiratory function and ventilatory response to hypercapnia. These changes lead to a reduction in the number and duration of hospital admissions and visits to emergency rooms, and an improvement in quality of life and survival.19,20 However, few studies have evaluated long-term survival, and behavioral differences between patients with and without OSAHS have not been analyzed, even though it has been shown that the latter can be treated safely with CPAP.21
The aim of this study was to determine the differences in progress between patients with OHS and those with OHS and OSAHS receiving home mechanical ventilation (HV), and to identify prognostic factors for survival.
MethodologyDesignThis was a prospective, longitudinal, observational study in a cohort of patients with a diagnosis of OHS seen in the Ventilatory Support Unit of our hospital over a 12-year period (from 1998 until 2010), who met all of the inclusion criteria and none of the exclusion criteria for participation in the HV program.
PopulationPatients with a confirmed diagnosis of OHS and a body mass index of over 30mg/m2, respiratory failure with daytime PaCO2 >45mmHg and nighttime >50mmHg, with or without associated OSAHS, were included. The patients entered the HV program in a stable state or after hospitalization for exacerbation, once clinical stability had been restored. Patients with obstructive disease defined as a FEV1/FVC% ratio <70%, neuromuscular disease with respiratory involvement and any other respiratory disease other than OHS were excluded. Although most of the definitions of the syndrome exclude significant change in ventilatory mechanics due to obesity, patients with any FVC value were included in this study, as it was thought that this would allow better assessment of the treatment.
Study GroupsSubjects were divided into 2 groups, according to nocturnal polysomnographic results: group 1 (OHS) consisting of patients with an apnea–hypopnea index (AHI) ≤5, and group 2 (OHS–OSAHS), consisting of patients with AHI >5.
MethodsBefore starting the ventilation program, a clinical questionnaire was completed for all patients, including symptoms, previous hospitalizations and intensive care admissions, spirometry data, baseline blood gases, early morning blood gases and a polysomnography study. Both spirometry and blood gases were determined according to Spanish Pulmonology and Thoracic Surgery Society guidelines.22 Sleep studies were carried out according to standard methods23 with duly validated 12-channel respiratory polygraphy (Apnoscreen II, Jaeger, Germany) or 18-channel polysomnography (Sleepscreen, Jaeger, Würzburg, Germany). Both devices measure airflow by thermistor and nasal cannula, producing automatic and manual readings.
Bilevel positive airway pressure (Respironics or Harmony BiPAP, Respironics, Lousville, USA) ventilators in ST mode with nasal masks were used. Positive expiratory pressure between 6 and 10cm H2O and positive inspiratory pressure of 16cm H2O increasing progressively until target efficacy objectives were achieved or a maximum pressure of 24cm H2O was reached. Initial support respiratory frequency was 16 breaths per minute, with an inspiration/expiration ratio of 0.4 or an inspiratory time of 1.5s. To facilitate patient adaptation, ventilation was initiated in 2-h periods during the day, with oxygen saturation monitoring and PaCO2 determination from blood gases at the beginning and at the end of the session. When oximetry showed persistent desaturation, oxygen supplements were added and the support pressure levels were adjusted according to final blood gas levels. Ventilation was considered to be effective when PaCO2 remained under 45mmHg or fell from baseline value by more than 5mmHg, with a mean oxygen saturation greater than 90%. After daytime adaptation, nighttime adaptation was undertaken. The patients’ oxygen saturation was monitored by oximetry and early morning blood gas levels. Patients with OHS–OSAHS underwent a second partially supervised polysomnography with the ventilator to adjust positive expiratory and inspiratory pressure in order to ensure control of nocturnal apnea and hypopnea events.
During the first year of follow-up, patients were seen every 3 months. Subsequently, they attended yearly check-ups throughout their participation in the HV program. In all of the check-ups, clinical data on symptoms, exacerbations, admissions, weight changes and side effects were recorded. Compliance was evaluated with a timer and lung function tests, arterial blood gases breathing room air, nocturnal oximetry under ventilation and nocturnal blood gases, also under ventilation, were determined. Ventilation was considered appropriate if nocturnal PaCO2 under ventilation was lower than 50mmHg, pH greater than 7.35 and oxygen saturation greater than 90% for 90% of the night. In patients with OHS/OSAHS who had basal PaCO2 of less than 45mmHg one year after starting ventilation, nocturnal blood gases without ventilation were determined; if this figure was maintained, treatment was switched to CPAP.
Statistical AnalysisA descriptive analysis of qualitative variables, expressed as absolute frequencies and percentages, was performed, while quantitative variables were presented as means and standard deviations (SD). For variables that did not show normal distribution or for which numbers were small (n<15), data are presented as median, interquartile range and minimum and maximum. Student's t-test was performed for simultaneous comparison of 2 groups. To analyze changes during patient follow-up at 1, 3, 5 and 8 years, Student's repeated measures t-test or Wilcoxon's test were used. When the size of the groups to be compared was small or they were heterogeneous, non-parametric statistical tests were used. For qualitative variables, Pearson's Chi-squared test was used to measure the association and compare proportions. When the groups were small, Fisher's exact test was used. Progression to death was studied using Kaplan–Meier tests and groups were compared with the log-rank test. The relative risk of survival was estimated using a Cox regression model.
ResultsInitial DataOver the 12-year period, 153 obese patients with chronic hypercapnia were included in the HV program. Of these, 70 also had airway obstruction and were excluded, leaving 83 patients: 60 women (72.3%) and 23 men (27.7%). Patients had lung function tests indicative of restrictive ventilatory defect with mean FVC of 65.9% (SD 19) and FEV1 of 66.3% (SD 19). Arterial blood gases showed overall respiratory failure, with a mean PaO2 of 52.3mmHg (SD 16.5) and mean PaCO2 of 63.6mmHg (SD 15.6).
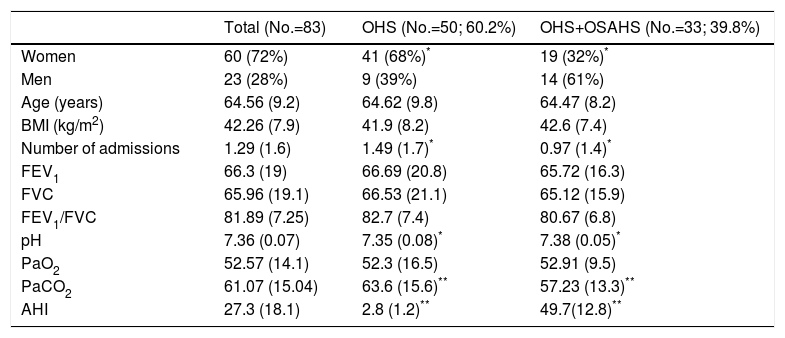
Fifty patients (60.2%) were included in group 1 (OHS without OSAHS) and 33 (39.8%) in group 2, (OHS with OSAHS). Clinical and functional characteristics are listed in Table 1. In the OHS group, 82% of subjects were women, compared to 57.6% in the OHS–OSAHS group (P<.05). PaCO2 in the OHS group was significantly higher than the OHS–OSAHS group (P<.01) and the former also had a higher number of hospitalizations per exacerbation (P<.05).
Patient Characteristics at Time of Inclusion.
| Total (No.=83) | OHS (No.=50; 60.2%) | OHS+OSAHS (No.=33; 39.8%) | |
|---|---|---|---|
| Women | 60 (72%) | 41 (68%)* | 19 (32%)* |
| Men | 23 (28%) | 9 (39%) | 14 (61%) |
| Age (years) | 64.56 (9.2) | 64.62 (9.8) | 64.47 (8.2) |
| BMI (kg/m2) | 42.26 (7.9) | 41.9 (8.2) | 42.6 (7.4) |
| Number of admissions | 1.29 (1.6) | 1.49 (1.7)* | 0.97 (1.4)* |
| FEV1 | 66.3 (19) | 66.69 (20.8) | 65.72 (16.3) |
| FVC | 65.96 (19.1) | 66.53 (21.1) | 65.12 (15.9) |
| FEV1/FVC | 81.89 (7.25) | 82.7 (7.4) | 80.67 (6.8) |
| pH | 7.36 (0.07) | 7.35 (0.08)* | 7.38 (0.05)* |
| PaO2 | 52.57 (14.1) | 52.3 (16.5) | 52.91 (9.5) |
| PaCO2 | 61.07 (15.04) | 63.6 (15.6)** | 57.23 (13.3)** |
| AHI | 27.3 (18.1) | 2.8 (1.2)** | 49.7(12.8)** |
FEV1: forced expiratory volume in first second; FVC: forced vital capacity; AHI: apnea–hypopnea index; BMI: body mass index; PaO2: pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood; OHS: obesity–hypoventilation syndrome: OSAHS: obstructive sleep apnea–hypopnea syndrome.
Values expressed as n (percentage) or mean (standard deviation). FEV1 and FVC expressed as percentages of the predicted value. PaO2 and PaCO2 expressed in mmHg.
Mean time of continuing ventilation was 7.44 years (95% confidence interval [95% CI]: 6.22–8.65). In 21 of the 83 patients (25%), ventilation was discontinued: 12 due to improvement and 9 due to withdrawal or too few hours of use (mean 5.7±1.3h/night, while good compliance was considered to be BiPAP used for over 4h/night). Of the 12 patients who improved, 9 belonged to the OHS–OSAHS group and switched to CPAP, and 3, who improved clinically due to weight loss, were in the OHS group.
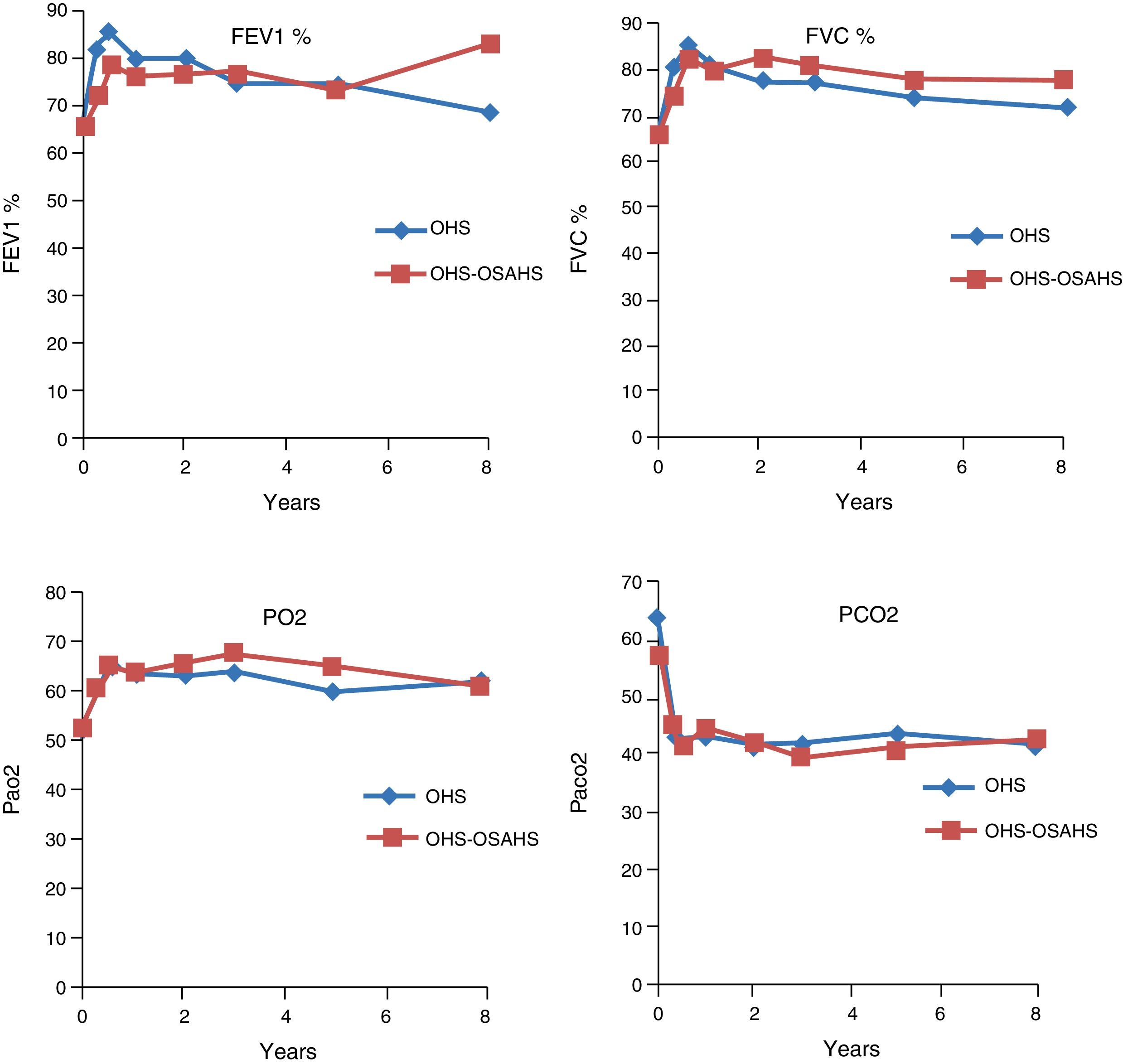
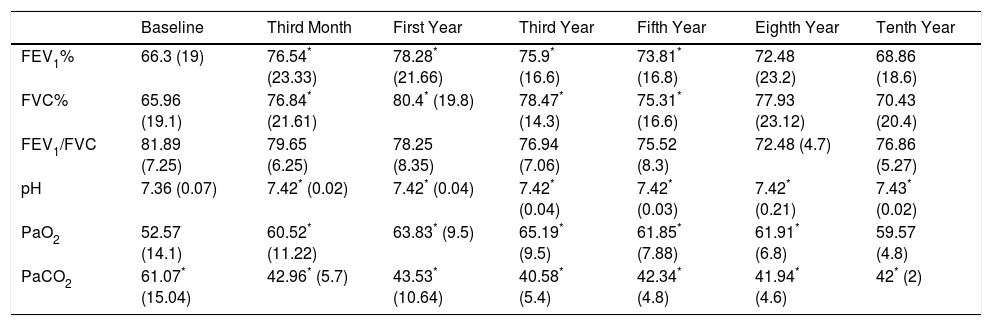
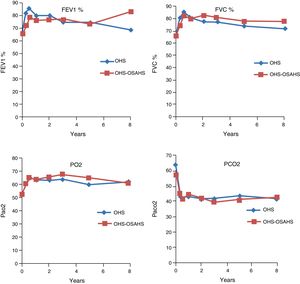
Course of Lung Function TestsIn both study groups, a significant improvement in both FEV1 and in FVC had been observed by month 3. This was maintained over the first, third, fifth, eighth and tenth year of treatment, although in the last 2 evaluation points the improvement was not significant, due to the small number of patients. With regard to the blood gas figures, there was also a significant improvement in PaO2 values, as well as a significant decrease in PaCO2 (Table 2). No differences were found between groups in the course of lung function and blood gas parameters (Fig. 1).
Evolution of Respiratory Function Tests and Blood Gas Levels at 3 Months and 1, 3, 5, 8 and 10 Years in the Pooled Population.
| Baseline | Third Month | First Year | Third Year | Fifth Year | Eighth Year | Tenth Year | |
|---|---|---|---|---|---|---|---|
| FEV1% | 66.3 (19) | 76.54* (23.33) | 78.28* (21.66) | 75.9* (16.6) | 73.81* (16.8) | 72.48 (23.2) | 68.86 (18.6) |
| FVC% | 65.96 (19.1) | 76.84* (21.61) | 80.4* (19.8) | 78.47* (14.3) | 75.31* (16.6) | 77.93 (23.12) | 70.43 (20.4) |
| FEV1/FVC | 81.89 (7.25) | 79.65 (6.25) | 78.25 (8.35) | 76.94 (7.06) | 75.52 (8.3) | 72.48 (4.7) | 76.86 (5.27) |
| pH | 7.36 (0.07) | 7.42* (0.02) | 7.42* (0.04) | 7.42* (0.04) | 7.42* (0.03) | 7.42* (0.21) | 7.43* (0.02) |
| PaO2 | 52.57 (14.1) | 60.52* (11.22) | 63.83* (9.5) | 65.19* (9.5) | 61.85* (7.88) | 61.91* (6.8) | 59.57 (4.8) |
| PaCO2 | 61.07* (15.04) | 42.96* (5.7) | 43.53* (10.64) | 40.58* (5.4) | 42.34* (4.8) | 41.94* (4.6) | 42* (2) |
FEV1: forced expiratory volume in first second; FVC: forced vital capacity; PaO2: pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood.
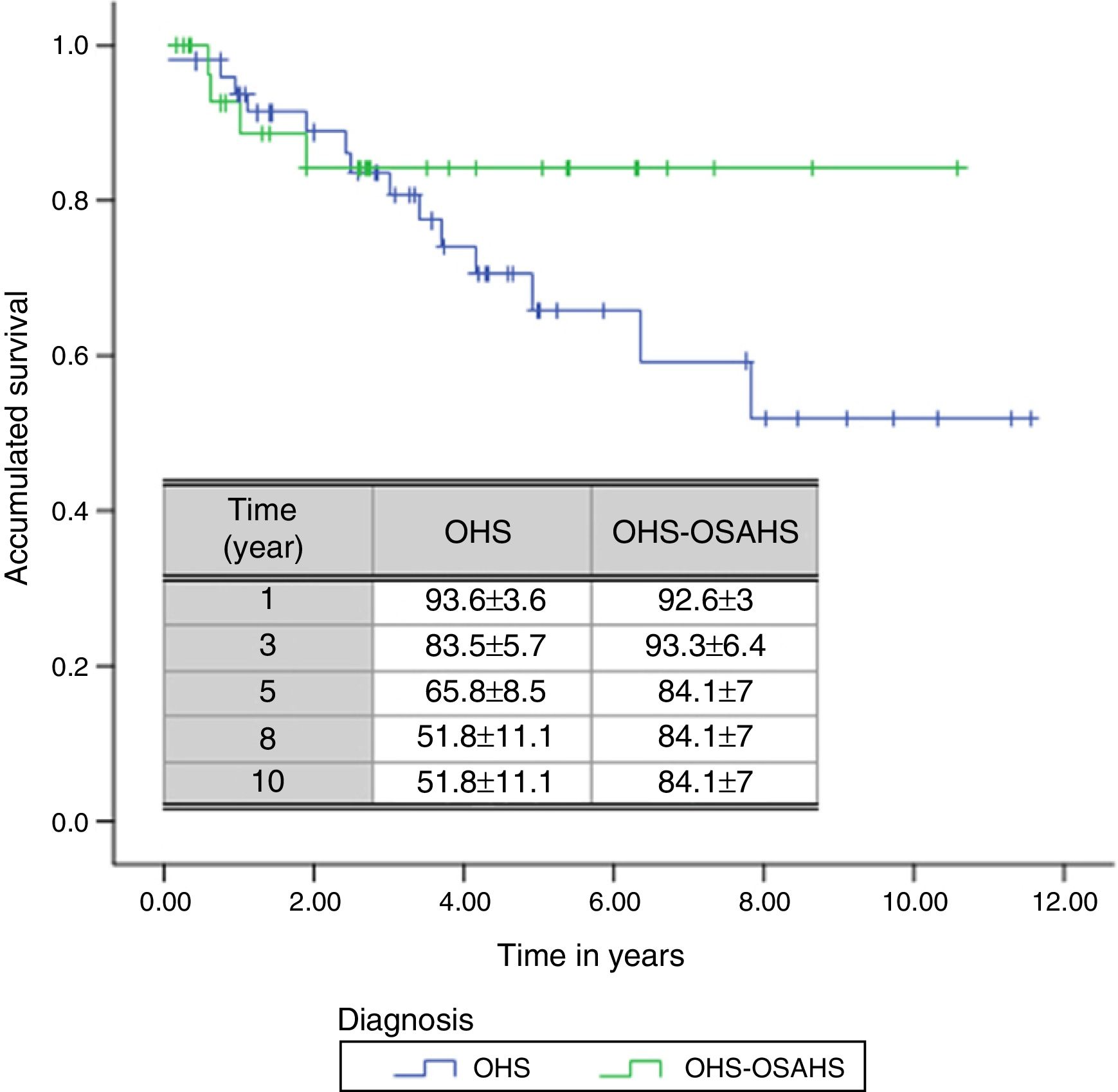
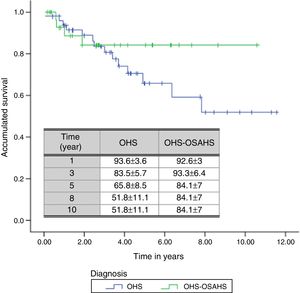
A total of 18 deaths (21.7% of all patients) were recorded during the follow-up period. The mean estimated survival time was 8.47 years, with a 95% CI of between 7.27 and 9.67 years. The probability of survival in the first, third, fifth, eighth and tenth year of follow-up was 93.3±3.1, 83.7±4.5, 71.9±6, 60.11±9.4 and 60.11%±9.4%, respectively.
With regard to diagnostic subgroups, 14 patients (28%) in group 1 (OHS) died, while in group 2, there were 4 deaths (12.1%). Although mean survival time of patients with OHS–OSAHS (9.07 years, 95% CI 7.71–10.45) was longer than that of patients with pure OHS (7.92, 95% CI 6.4–9.4), the difference was not statistically significant. Survival after the third year was greater in patients with OSH–OSAHS, but no significant differences were found between groups in accumulated survival due to the greater survival of the OHS group in the first year (Fig. 2).
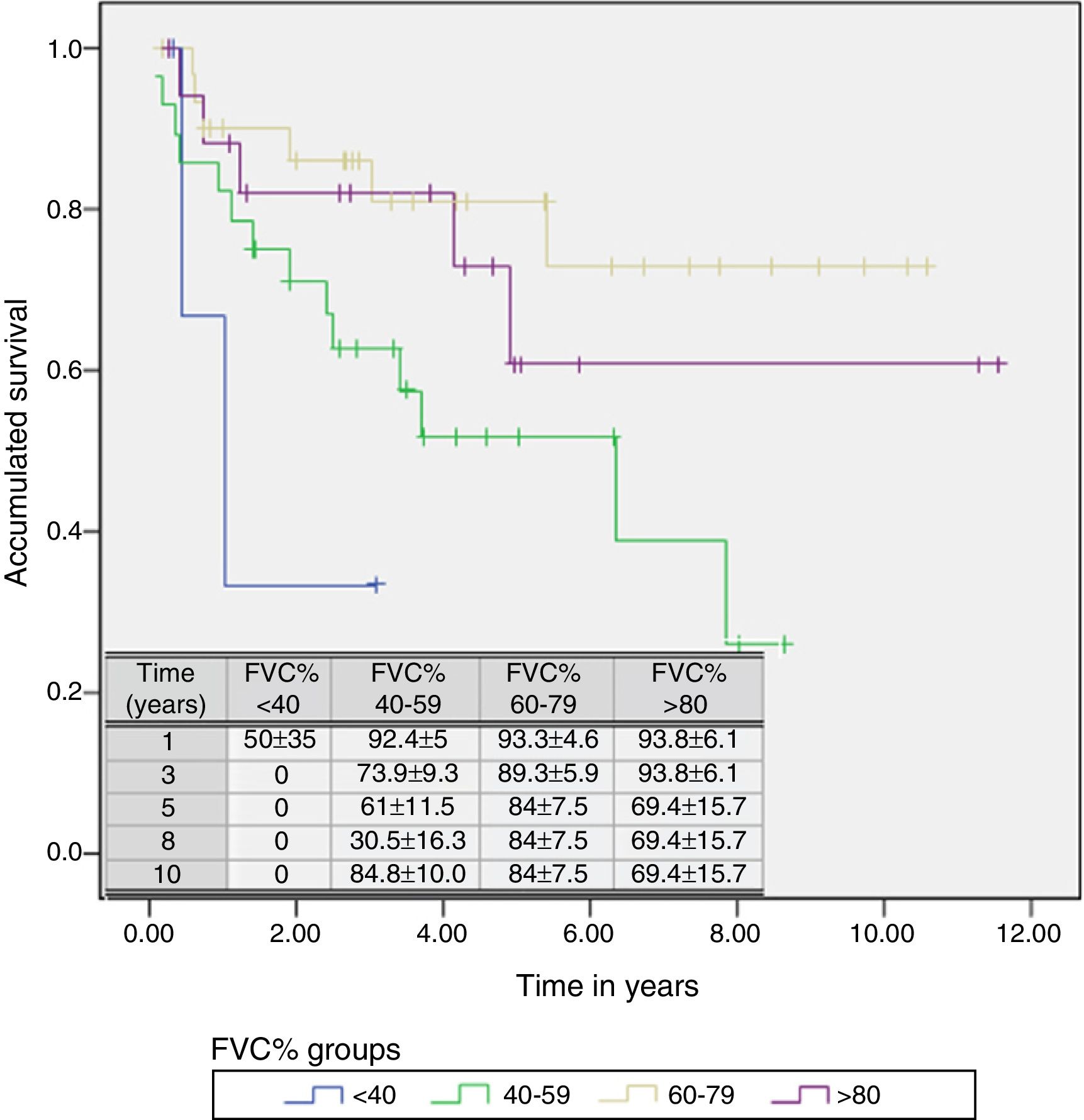
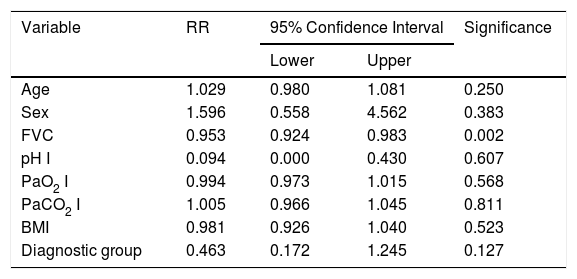
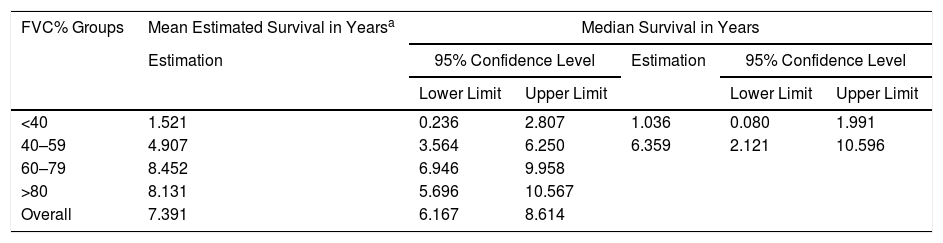
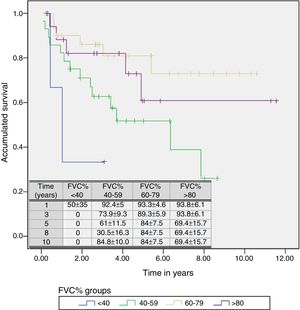
A Cox regression analysis aimed at studying risk factors showed that only FVC was a predictive factor for mortality, while the other factors included in the analysis (age, sex, BMI, PaO2, PaCO2 and diagnostic group) had no predictive value (Table 3). Stratification according to percent predicted FVC into 4 patient subgroups (<40%, 40%–59%, 60%–79% and >80%) showed that mean estimated survival increased in line with FVC, and was significantly greater in patients with FVC between 60% and 79% than in those with FVC less than 40% or between 40% and 60% (Table 4). The accumulated survival analysis (Fig. 3) also found an increased probability of survival that was greater for patients with higher FVC, although because of the low number of patients in the various subgroups, the difference was only significant for those in the group with FVC between 60% and 79% vs patients with FVC between 40% and 59%.
Predictive Factors for Risk of Death. Results of the Cox Regression Analysis.
| Variable | RR | 95% Confidence Interval | Significance | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.029 | 0.980 | 1.081 | 0.250 |
| Sex | 1.596 | 0.558 | 4.562 | 0.383 |
| FVC | 0.953 | 0.924 | 0.983 | 0.002 |
| pH I | 0.094 | 0.000 | 0.430 | 0.607 |
| PaO2 I | 0.994 | 0.973 | 1.015 | 0.568 |
| PaCO2 I | 1.005 | 0.966 | 1.045 | 0.811 |
| BMI | 0.981 | 0.926 | 1.040 | 0.523 |
| Diagnostic group | 0.463 | 0.172 | 1.245 | 0.127 |
FVC: forced vital capacity; BMI: body mass index; PaO2: pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood; RR: relative risk.
PaO2 I, PaCO2I and pH I are baseline values determined when the patient started home ventilation.
Mean Estimated Survival and Median Survival Stratified by Forced Vital Capacity Expressed in Percentage.
| FVC% Groups | Mean Estimated Survival in Yearsa | Median Survival in Years | ||||
|---|---|---|---|---|---|---|
| Estimation | 95% Confidence Level | Estimation | 95% Confidence Level | |||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||
| <40 | 1.521 | 0.236 | 2.807 | 1.036 | 0.080 | 1.991 |
| 40–59 | 4.907 | 3.564 | 6.250 | 6.359 | 2.121 | 10.596 |
| 60–79 | 8.452 | 6.946 | 9.958 | |||
| >80 | 8.131 | 5.696 | 10.567 | |||
| Overall | 7.391 | 6.167 | 8.614 | |||
FVC: forced vital capacity.
In this study, HV was found to be effective in the treatment of patients with OHS. It was associated with blood gas and functional improvement and with increased survival. Results are better in subjects with OHS–OSAHS, although the differences between these 2 groups are not significant and the only predictive factor for survival was good FVC at outset.
Historically, ventilatory support for patients with OHS was first used with concomitant OSAHS. CPAP was used initially and, if this was insufficient to control hypoventilation, positive pressure ventilation was applied using either bilevel pressure or volume-controlled ventilators.14–16 Since then, long-term NIV results have been analyzed in numerous cohort studies of OHS patients,18,24–27 all of which have reported improvement in blood gas levels, and OHS is now among the indications given in guidelines and recommendations for the use of HV.17 To date, only one clinical trial has been published in which the results of NIV are compared with conventional oxygen therapy. This is a small randomized study in 36 patients with very mild hypercapnia and a follow-up period of only one month. However, despite these limitations, improvement in sleep structure and gas exchange were greater than in the control group.28
All NIV cohort studies in patients with OSH show an improvement in gas exchange. This was also found in this study, in which correction of hypercapnia was seen from the beginning of treatment, an improvement that persisted for as long as 10 years in both OSH and OSH-OSAHS patients. This improvement has been attributed to multiple mechanisms, including recovery of sensitivity to hypercapnia, respiratory muscle rest and improvement in ventilatory mechanics.8,19,20
In patients with OHS, the use of NIV is associated with an increase in respiratory minute volume (VE) and occlusion pressure in response to hypercapnia.24,29 A previous study performed by our group in a small series of patients also found that the use of NIV in patients with OHS was associated with an increase in the VE/PaCO2 and P0.1/PaCO2 slope,24 but these variables were not available in most of the patients in the cohort analyzed here, so these findings cannot be confirmed. Changes in PaO2, in addition to reflecting variations in ventilation, may also be related to an improvement in the level of pulmonary hypertension and right ventricular overload that frequently develop in these patients, and, according to some recent studies, may be reversed with the use of HV.30,31 Two important aspects to remember when considering the improvement in blood gas levels are good ventilatory support during administration and adherence to treatment. Our working procedures include nocturnal arterial blood gas monitoring for evaluating the efficacy of the ventilation, so all patients were subject to nocturnal monitoring, both at the start of ventilation and during regular check-ups, the results of which were used to adjust the ventilator parameters. Treatment adherence is known to be a prerequisite for normalizing blood gas levels, with reports indicating an association between the number of hours of ventilation and the degree of PaO2 reduction, and only around 4.5h of ventilation can achieve significant blood gas level improvements.32 The patients in this study received more than 4h of ventilatory support, a factor that also contributed to the good results obtained.
With regard to changes in ventilatory mechanics, the literature is contradictory, and although the study published by Heinemann et al.26 showed that NIV in OSH patients was associated with an increase in inspiratory capacity and total lung capacity, this has not been confirmed by other authors.20,25 Although lung volumes were not measured in this study, a sustained increase in FVC was found. Other factors cannot be ruled out, but similarly to Heinemann et al., high support pressures were used in this study, which could have assisted in resolving possible microatelectasis, thus improving respiratory mechanics. Muscle rest provided by NIV may also contribute to the improvement in lung volumes,33 but since pressures were not measured in this study no conclusions could be drawn in this regard.
The most important aspect of this study is the impact of HV on mortality. Few studies have analyzed the survival of patients with OHS treated with HV for periods longer than 5 years.28,34 Our results confirm the findings of those studies, with survival rates of over 90% in the first year and 70% in the fifth. We also had good results for 8- and 10-year survival, data that has not yet been reported by other researchers. Unlike other authors,27,34 we did not find that the degree of hypoxemia or hypercapnia or pH levels had a prognostic value for patient survival, but the difference is probably due to the fact that our patients were very closely assessed before inclusion in an HV program, i.e., their situation was already stable.
None of the previous studies analyzed the impact of OSAHS associated with OHS on patient survival. In our study, after the first year of ventilation, patients with OSAHS showed a clear trend toward improved survival, although this was not statistically significant. Since patients with OHS initially had a higher level of hypoventilation than those with OHS–OSAHS, correction of airway instability in the latter may have a more favorable effect on gas exchange, but differences in other fundamental physiological aspects cannot be ruled out.
One important and hitherto unreported factor analyzed is the impact of changes in ventilatory mechanics on the clinical course. In this study, the only factor with predictive value for survival was FVC: the lower the FVC, the less chance of survival. It can be assumed that in patients with more compromised ventilation at the time of diagnosis, gas exchange alterations would already have caused irreversible structural changes that would explain their poorer progress, even after an initial improvement in blood gas levels. Moreover, as data on the cause of death are not available, we cannot rule out that patients with greater restriction did not also have greater associated comorbidity.
Interestingly, although most of the OHS patients in the series published to date also had OSAHS, these subjects were a minority in our study. This may be because our population had a greater level of hypercapnia that those of other studies, and patients were referred for respiratory failure and not because of suspected sleep apnea.
One of the most important limitations of this study is the lack of data related to the impact of comorbidity on the clinical course and survival of patients, since, although follow-up was prospective, this information was not included when the statistical analysis plan was designed. Nor is it possible to analyze which type of ventilator would be more effective or, more importantly, whether some patients could have been treated from the outset with CPAP instead of NIV.
Although some authors claim that in extremely obese patients the use of volume-cycled ventilators may be necessary, all published series have reported satisfactory results with bilevel ventilators with backup respiratory rate for avoiding central apnea events35; the efficacy of this technology is supported by our results. It has been suggested recently that the use of bilevel volume-assured pressure support may have additional advantages. However, the available data are contradictory and although 2 clinical trials aimed at evaluating this technology have been performed, very few patients were included and results cannot be considered conclusive.36,37 As for the question of whether patients with OSAHS could have been effectively treated with CPAP, all these patients were included in the HV program according to protocol, so this aspect could not be analyzed. What was observed was that, after correction of initial hypoventilation, some patients could be switched to CPAP; this finding has already been published by our group in a previous study38 and was also reported in the series published by Pérez de Llano et al.21 The problem is that, although treatment algorithms recommend starting CPAP therapy in stable, acidosis-free patients, in most studies treatment is initiated with BiPAP, whether in the acute or stable phase. Only after blood gas levels have improved is a switch to CPAP considered in patients with OSAHS. To date, only one short-term randomized clinical trial in a limited number of patients with an inclusion criterion of only mild PaO2 and PaCO2 alteration has been published. The authors conclude that both treatments are equally effective.39 Enough data is currently available to support the recommendation that patients with mild gas exchange alterations associated with moderate or severe OSAHS begin treatment with CPAP, while BiPAP would be the treatment of choice for patients with clearly predominant nocturnal hypoventilation.4,8,20,40 Additional clinical trials are necessary to position one or the other technique.
To conclude, our results confirm that the use of HV in OHS patients with or without OSAHS is very effective for correcting gas exchange alterations and improving ventilatory mechanics, and survival rates can exceed those reported in other populations classically considered candidates for this therapy. Two questions, however, remain: which is the ideal patient population for this treatment, and in which patients can CPAP from outset assure the same level of safety and efficacy, assuming clinical stability in both cases.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Ojeda Castillejo E, de Lucas Ramos P, López Martin S, Resano Barrios P, Rodríguez Rodríguez P, Morán Caicedo L, et al. Ventilación mecánica no invasiva en pacientes con síndrome de obesidad-hipoventilación. Evolución a largo plazo y factores pronósticos. Arch Bronconeumol. 2015;51:61–68.