Our objective was to evaluate whether the number and volume of surgical lung biopsies (SLBs) influence the diagnosis of diffuse interstitial lung disease (ILD).
MethodsRetrospective study of SLB for suspected ILD in patients from the Mayo Clinic from January 2002 to January 2010. Data were collected in the institution and analyzed.
Results311 patients were studied. Mean number of biopsies was 2.05 (SD 0.6); 1 biopsy in 50 (16%), 2 in 198 (63.7%), 3 in 59 (19%) and 4 in 4 (1.3%). Histopathologic diagnosis was: definitive (specific): 232 (74.6%), descriptive (non-specific): 76 (24.4%), no diagnosis: 3 (1%). After excluding patients without diagnosis (n=3), there were 50 patients with only 1 biopsy, 196 with 2 and 62 with 3 or 4; the definitive diagnostic yield was similar in all 3 groups (37/50; 74%, 150/196; 77%, and 45/62; 73%) (Chi-square, P value .8). The propensity score analysis between patients with 1 SLB and patients with more than 1 SLB also showed no difference in diagnostic yield. Regarding the volume of biopsies, mean total volume was 34.4cm3 (SD 46): 41.2cm3 (3 cases) in patients with no diagnosis; 33.6cm3 (232 cases, SD 47) in patients with specific diagnosis; and 36.6cm3 (76 cases, SD 44) in patients with descriptive diagnosis. Biopsy volume had no influence on histopathology yield (ANOVA, P value .8).
ConclusionsThe number and volume of the biopsy specimens in SLB did not seem to influence diagnosis. Based on our results, we believe a single sample from a representative area may be sufficient for diagnosis. Randomized prospective trials should be performed to optimize SLB for ILD.
Nuestro objetivo fue evaluar si el número y el volumen de las biopsias pulmonares quirúrgicas (BPQ) influyen en el diagnóstico de la enfermedad pulmonar intersticial difusa (EPID).
MétodosEstudio retrospectivo de BPQ por sospecha de EPID en los pacientes de la Clínica Mayo desde enero de 2002 hasta enero de 2010. Los datos se recogieron y analizaron en la institución.
ResultadosSe analizaron 311 pacientes. El número medio de biopsias fue de 2,05 (DE 0,6); una biopsia en 50 (16%), dos en 198 (63,7%), tres en 59 (19%) y cuatro en 4 (1,3%). El diagnóstico histopatológico fue: definitivo (específico) en 232 (74,6%), descriptivo (no específico) en 76 (24,4%) no hubo diagnóstico en 3 (1%). Tras excluir a los pacientes sin diagnóstico (n=3), hubo 50 pacientes con solo una biopsia, 196 con dos y 62 con tres o cuatro. El rendimiento de diagnóstico definitivo fue similar en los tres grupos (37/50 [74%], 150/196 [77%] y 45/62 [73%]). El valor p de Chi-cuadrado fue 0,8. El análisis del índice de propensión entre pacientes con una BPQ y pacientes con más de una BPQ tampoco mostró ninguna diferencia en el rendimiento diagnóstico. En cuanto al volumen de las biopsias, el volumen total medio fue 34,4cm3 (DE=46). En los pacientes sin diagnóstico, 41,2cm3 (3 casos), en pacientes con diagnóstico específico, 33,6cm3 (232 casos [DE=47]) y en pacientes con un diagnóstico descriptivo: 36,6cm3 (76 pacientes [DE=44]). El volumen de la biopsia no influyó en el rendimiento de la histopatología (ANOVA, p=0,8).
ConclusionesEl número o el volumen de las muestras de biopsia quirúrgica no parecen influir en el diagnóstico. Según nuestros resultados creemos que una sola muestra de un área representativa puede ser suficiente para el diagnóstico. Se deben realizar ensayos prospectivos aleatorizados para optimizar la BPQ en las EPID.
Interstitial lung disease (ILD) is a generic term representing a heterogeneous group of lung diseases classified together due to several common features.1 Idiopathic pulmonary fibrosis is the most common of all ILD.2
Both the current classification of ILD and guidelines recommend surgical lung biopsy (SLB) for definite diagnosis of ILD, but encourage physicians to balance the benefit carefully against the risks of performing the surgery.3,4
Indeed, the decision to perform SLB in these patients is based on the likelihood that pathologic examination of the tissue obtained will yield relevant features for a confident diagnosis or specific information about the cause of the disease process.5,6
Therefore, it is crucial to ascertain the optimal number and volume of SLBs in order to improve diagnostic yield. Few studies have attempted to identify these factors. In order to answer this question, we ran a propensity score analysis on our SLB patients to match patients with 1 biopsy and those with more than 1 to ascertain the optimal number of samples needed to obtain a specific diagnosis. We also analyzed the influence of sample volume on the diagnostic yield.
MethodsRetrospective study of data collected from the Mayo Clinic SLB database between 1 January 2002 and 31 January 2010. The Institution's patient data access protocols were followed for the sole purpose of scientific investigation and disclosure. Inclusion criteria were patients with radiological suspicion of ILD who were candidates diagnostic SLB. Patients with a solitary pulmonary nodule/mass or with other focal pulmonary processes were not included. All patients had been previously investigated with high resolution chest computerized tomography (HRCT), and most of them had undergone bronchoscopy and pulmonary function tests. Pulmonary function tests were not performed in ICU cases and in patients whose clinical state was judged excellent. SLB sample sites were selected on the basis of HRCT images.
Variables were: age, sex, smoking history, comorbidities, preoperative diagnostic tests, pulmonary function tests, admission characteristics, type of operation, intraoperative complications, number of biopsies, volume of biopsies, length of stay, postoperative complications, postoperative morbidity and mortality, and histopathologic diagnosis of each sample. Diagnostic yield and non-diagnostic yield groups were formed on the basis of specific diagnosis reached as a result of SLB.
To minimize selection bias, a propensity score analysis taking into account several baseline and surgical characteristics was performed to create 2 well-matched groups of patients receiving 1 or more biopsies.7 The procedure yielded 2 well matched groups of 50 patients each.
Numeric variables were compared by means of the Mann Whitney test and categorical variables were compared by means of the Chi-square test or Fisher's exact test, as appropriate. All tests were two-tailed with a significance level of 0.05. Statistics were analyzed on Stata 9.0 statistical software (Stata Corp., College Station, TX).
ResultsA total of 311 patients were analyzed. The average age at diagnosis was 60.9 (Standard Deviation [SD] 14), range 18–91. Sex distribution was 164 males (52.7%) and 147 females (47.3%). Smoking history showed 143 never smokers (46%), 30 current smokers (9.6%) and 138 past smokers (44.4%). Major comorbidities were present in 91 patients (29.3%): ischemic heart disease in 67 (21.5%), arrhythmia in 24 (7.7%) and chronic renal failure in 23 (7.4%). Ten patients (3.2%) had previously undergone a transplant, 4 bone marrow transplantations and 6 solid organ transplantations. Seventy-eight patients (25%) were on steroids, and 26 (8.4%) were immunosuppressed at the time of the SLB. HRCT had been performed in all study patients prior to SLB.
Bronchoscopy had been performed in 202 patients (65%), and pulmonary function tests in 259 patients (83.3%). Mean values of the latter were: FEV1%: 70.2 (SD 16.3), FVC%: 70.1 (SD 16), FEV1/FVC: 79.8 (SD 8.8), DLCO%: 52.1 (SD 14.7). Regarding admission characteristics, 292 patients (93.9%) were hospitalized and 19 patients (6.1%) were in the ICU before the LB.
Type of operation was open LB in 32 patients (10.3%) and VATS in 279 patients (89.7%). Reason for open LB were adhesions in 27 (84%) and inability to tolerate single lung ventilation in 5 (16%). No intraoperative complications were reported.
Mean length of stay was 2.5 days. Twenty-five patients (8%) were transferred to another department after the SLB. There were postoperative complications in 36 patients (11.5%). The most frequent complications were: acute exacerbation of respiratory failure (26.1%), postoperative requirement of ICU (not already in ICU) (22.7%), requirement of intubation (not already intubated) (13.6%) and prolonged air leakage (>7 days) (10.2%). Mortality rates were: LB 30-day post-operative mortality, 28 patients (9%), LB 90-day mortality, 33 patients (10.6%).
Histopathologic diagnosis was definitive (specific) in 232 (74.6%), descriptive (non-specific) in 76 (24.4%) and there was no diagnosis in 3 patients (1%). Most frequent diagnoses were usual interstitial pneumonia (all the cases resulting in idiopathic pulmonary fibrosis) in 122 (39%), cryptogenic organizing pneumonia in 31 (10%) and respiratory bronchiolitis ILD in 16 (5%) (Table 1).
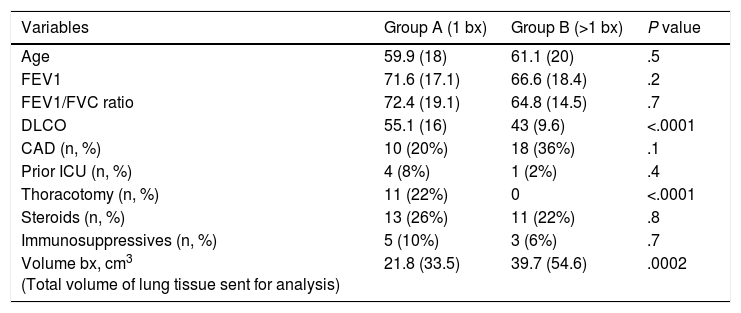
Diagnosis of Patients With Specific Histologic Diagnosis.
| Idiopathic pulmonary fibrosis/usual interstitial pneumonia | 122 (39.2 52.6%) |
| Cryptogenic organizing pneumonia | 31 (9.9 13.4%) |
| Respiratory bronchiolitis interstitial lung disease | 16 (5.1 7%) |
| Nonspecific interstitial pneumonia | 13 (4.2 5.6%) |
| Hypersensitivity pneumonitis | 12 (3.8 5.2%) |
| Lymphoma | 12 (3.8 5.2%) |
| Acute interstitial pneumonia | 10 (3.2 4.2%) |
| Sarcoidosis | 10 (3.2 4.2%) |
| Infection | 6 (1.9 2.6%) |
After excluding patients without diagnosis (n=3), there were 50 patients with only 1 biopsy, 196 with 2 and 62 with 3 or 4; the definitive diagnostic yield was similar in all 3 groups (37/50; 74%, 150/196; 77%, and 45/62; 73%) (Chi-square, P value .8). The propensity score analysis to match patients with 1 SLB (group A) and more than 1 SLB (group B) yielded 50 pairs. There was no difference in diagnostic yield between these 2 matched groups. No difference was noted either in terms of morbidity (group A: 8, group B: 5, P=.6) and 90-day mortality (group A: 5, group B: 6, P=1). Comparison between propensity matched groups is shown in Table 2. Regarding the volume of biopsies, mean total volume (sum of the volumes of all biopsies divided by the number of patients) was 34.4cm3 (SD 46). Volume of biopsies in patients with no diagnosis was 41.2cm3 (3 cases), with specific diagnosis was 33.6cm3 (232 cases, SD 47), and with descriptive diagnosis was 36.6cm3 (76 patients, SD 44). There was no influence of volume of biopsy on the yield of histopathology (ANOVA test, P value .8).
Comparison Between Propensity Matched Groups.
| Variables | Group A (1 bx) | Group B (>1 bx) | P value |
|---|---|---|---|
| Age | 59.9 (18) | 61.1 (20) | .5 |
| FEV1 | 71.6 (17.1) | 66.6 (18.4) | .2 |
| FEV1/FVC ratio | 72.4 (19.1) | 64.8 (14.5) | .7 |
| DLCO | 55.1 (16) | 43 (9.6) | <.0001 |
| CAD (n, %) | 10 (20%) | 18 (36%) | .1 |
| Prior ICU (n, %) | 4 (8%) | 1 (2%) | .4 |
| Thoracotomy (n, %) | 11 (22%) | 0 | <.0001 |
| Steroids (n, %) | 13 (26%) | 11 (22%) | .8 |
| Immunosuppressives (n, %) | 5 (10%) | 3 (6%) | .7 |
| Volume bx, cm3 (Total volume of lung tissue sent for analysis) | 21.8 (33.5) | 39.7 (54.6) | .0002 |
Results are expressed as mean±standard deviation unless otherwise indicated. Numeric variables compared by Mann Whitney test. Categorical variable compared by Fisher's exact test. Bx: surgical lung biopsy; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; DLCO: diffusing capacity for carbon monoxide; CAD: coronary artery disease; ICU: Intensive Care Unit.
ILD is a group of diseases with a great diversity of treatment options and prognosis.8 Generally, lung tissue is still required for the diagnosis of ILD in approximately one third of patients with no clearly defined environmental exposure or obvious systemic illness that frequently involves the lung.
Transbronchial lung biopsy (TBLB) has been used to obtain peripheral lung tissue for this purpose, and recently the use of cryoprobes for TBLB has become an alternative technique to increase diagnostic yield with larger and better quality biopsy samples.9 In the absence of improvements in these minimally invasive techniques, SLB is still required in many cases, but its role remains debatable. Despite the benefits obtained in the shift from open LB to VATS-LB, many clinicians are still reluctant to allow patients to undergo surgery without assurances that results will lead to a change in therapy for a significant number.10 In this study of SLB, a specific diagnosis was reached in 74.6% of cases, a rate we consider acceptable given that specific diagnosis rates in the literature vary from 34% to 98%.11,12 We believe it is remarkable that although more than 25% of cases received some anti-inflammatory treatment, the percentage of diagnostic yield was still high, a finding that reinforces the utility of surgical lung biopsy for ILD diagnosis.
Morbidity and mortality rates were 11.5% and 10.6% (90-day). These rates vary greatly in the literature, and this discordance reflects the diverse clinical characteristics of the patients included in surgical ILD series.13 The most common pathologic diagnosis obtained was usual interstitial pneumonia, resulting in all cases in idiopathic pulmonary fibrosis (39%). Epidemiologic studies of ILD report that idiopathic pulmonary fibrosis is the most frequent form of ILD, ranging from 25% to 38% depending on the series.14
The question of the most appropriate number of biopsies has been widely debated. Several authors suggest performing more than 1 biopsy: in their series with multiple samples, Monaghan et al.15 and Flaherty et al.16 obtained a diagnosis concordance of 87.5% and 74%, respectively. This was one of the reasons for suggesting taking more than 1 sample to reach a confident ILD diagnosis. Other authors, however, recommend taking just 1 sample from the most representative areas: Chechani et al.17 obtained 100% concordance and in a multicenter project involving 224 patients Fibla et al.18 reported a concordance of 97.2%. Both studies concluded that there was no need for multiple biopsy specimens when a radiographically representative region could be sampled in 1 biopsy. Based on our results, we also feel that 1 biopsy from a representative area containing both pathological and normal parenchyma is sufficient to provide a diagnosis. Both studies concluded that pathological concordance of the samples observed by the pathologists was high (100% and 97.2%). In our study we also observed a high concordance. Our results are based on the analysis of 311 patients which, when added to previous similar studies17,18 provide a large number of cases showing that taking a single sample from a representative region containing both pathological and normal parenchyma can be sufficient for diagnosis. All these studies, however, (including ours) are retrospective. Prospective randomized studies should be performed to confirm this observation.
The volume of samples taken is also debatable. Qureshi et al.19 contend that a good general rule is to select 2 or 3 samples of approximately 3cm×2cm×1cm, however Flint et al.20 conclude that a single (>2cm diameter) specimen obtained from a region of the most radiographically involved lobe provides adequate tissue for diagnostic purposes. In our series, despite considerable variation of biopsy sizes, difference in mean volume was not statistically significant in either the specific diagnosis or non-specific diagnosis group.
The limitations of this study are its retrospective nature, using data providing from a single institution, and the fact that specific diagnosis was not analyzed according to the exact number of biopsies.
The strengths of this study are that it addresses a specific subset of patients, as it includes only patients with ILD who are potential candidates for SLB. What it does not include are patients with pulmonary masses or nodules who are candidates for wedge resection for either diagnosis or treatment. Another strong point is that histopathologic processing and reading was standardized and conducted by the same pathologists.
In conclusion, ILD patients are particularly complex and should be treated by specialized teams including pulmonologists, radiologists, pathologists and surgeons. VATS-LB is a powerful and safe tool for the diagnosis of suspected DILD, giving a definite diagnosis for the majority of patients with a low morbidity rate, and the number and volume of biopsy specimens does not seem to influence diagnosis. Based on our results, we believe that a single sample from a representative area may be sufficient for diagnosis. Randomized prospective trials, ideally focused on specific entities, should be performed to optimize the surgical lung biopsy for ILD.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Fibla JJ, Brunelli A, Allen MS, Wigle D, Shen R, Nichols F, et al. ¿Influyen el número y volumen de las biopsias pulmonares en el rendimiento diagnóstico en la enfermedad pulmonar intersticial? Análisis mediante índice de propensión. Arch Bronconeumol. 2015;51:76–79.