There are no previous studies aimed at assessing the validity of the screening scales for depression and anxiety in adult patients with bronchiectasis.
AimsTo analyze the psychometric properties of Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory (BDI) and Hamilton Anxiety Scale and to evaluate the concordance for the diagnosis of depression and anxiety between these screening scales and the structured clinical interview in adult patients with bronchiectasis.
MethodCross sectional study. 52 patients with bronchiectasis completed HADS, BDI and Hamilton Anxiety Scale; afterwards, were individually interviewed by a mental health care professional using the structured Mini International Neuropsychiatric Interview (MINI), which evaluates for depression and anxiety according to DSM-IV criteria.
ResultsBased on MINI, 18 subjects (34.6%) had a diagnosis of depression and 25 (48.1%) had anxiety. Optimal cut-off values to detect depression were ≥9 for the HADS-D (sensitivity 0.833, specificity 0.971, AUC 0.962 [95% CI 0.918–1]), and 17 for BDI (sensitivity 0.889, specificity 0.912, AUC 0.978 [95% CI 0.945–1]). Optimal cut-off values to detect anxiety were ≥4 for the HADS-A (sensitivity 0.960, specificity 0.593, AUC 0.833 [95% CI 0.723–0.943]), and 17 for Hamilton Anxiety Scale (sensitivity 0.800, specificity 0.852, AUC 0.876 [95% CI 0.781–0.970]).
ConclusionThe self-rating screening scales HADS, BDI and Hamilton Anxiety Scale are reliable tools to screen for depression and anxiety in adult patients with bronchiectasis. However, the use of specific cut-off values may improve the diagnostic accuracy of the previous scales in this specific group of patients.
No existen estudios previos dirigidos a la evaluación de las escalas de detección de la depresión y de la ansiedad en pacientes adultos con bronquiectasias.
ObjetivosAnalizar las propiedades psicométricas de la escala de ansiedad y depresión hospitalaria (HADS, por sus siglas en inglés), el inventario de depresión de Beck (BDI, por sus siglas en inglés) y la escala de ansiedad de Hamilton, y evaluar la concordancia para el diagnóstico de la depresión y la ansiedad entre estas escalas de detección y la entrevista clínica estructurada en pacientes adultos con bronquiectasias.
MétodoEstudio transversal. Cincuenta y dos pacientes con bronquiectasias completaron la HADS, el BDI y la escala de ansiedad de Hamilton; posteriormente, un psiquiatra profesional de la salud mental les entrevistó individualmente utilizando la entrevista estructurada denominada Minientrevista neuropsiquiátrica internacional (MINI), que evalúa la depresión y la ansiedad siguiendo los criterios del DSM-IV.
ResultadosBasándonos en la MINI, 18 sujetos (el 34,6%) fueron diagnosticados de depresión y 25 de ellos (el 48,1%) presentaba ansiedad. Los valores de corte óptimos para detectar depresión fueron ≥9 para la HADS-D (sensibilidad: 0,833; especificidad: 0,971; ABC: 0,962 [IC 95%: 0,918-1]) y 17 para el BDI (sensibilidad: 0,889; especificidad: 0,912; ABC: 0,978 [IC 95%: 0,945-1]). Los valores de corte óptimos para detectar ansiedad fueron ≥4 para la HADS-A (sensibilidad: 0,960; especificidad: 0,593; ABC: 0,833 [IC 95%: 0,723-0,943]) y 17 para la escala de ansiedad de Hamilton (sensibilidad: 0,800; especificidad: 0,852; ABC: 0,876 [IC 95%: 0,781-0,970]).
ConclusiónLas escalas de autoevaluación HADS, BDI y la escala de ansiedad de Hamilton son herramientas fiables para detectar la depresión y la ansiedad en pacientes adultos con bronquiectasias. Sin embargo, el uso de valores de corte específicos puede mejorar la precisión diagnóstica de las escalas anteriores en este grupo concreto de pacientes.
Depression and anxiety are prevalent in the general population and represent a significant public health problem.1–3 These psychiatric disorders are often under-diagnosed, occur comorbidly with chronic illnesses and are associated with worse adherence to prescribed treatments and increased health care utilization and costs.4–8
The gold standard for diagnosing psychiatric disorders is a structured clinical interview, such as the Structured Clinical Interview for DSM-IV.9 However, structured clinical interviews require administration by trained health professionals, are time-consuming and difficult to apply in routine clinical practice. Reliable, valid and easily applicable screening instruments are needed to identify patients with depression and anxiety.
The Hospital Anxiety and Depression Scale (HADS)10 and the Beck Depression Inventory (BDI)11 have proved to be reliable depression screening instruments in general practice and in medical patients,12,13 and they have been well validated for use in the Spanish population.14,15 The Hamilton Anxiety Scale16 is a scale developed to measure the severity of anxiety symptoms, and it has been validated for use in the Spanish population.17
Bronchiectasis is the end result of several different diseases, managed in similar ways, but which lead to pulmonary infections, loss of lung function and worsening of health related quality of life.18,19 Recent studies have shown that patients with bronchiectasis have a higher prevalence of symptoms of depression (between 20 and 30%) and anxiety (around 40%) than healthy subjects.20–23 However, these studies used screening scales such as HADS and BDI, and not a psychological evaluation performed by a mental health professional, to evaluate the presence of symptoms of depression and anxiety.
There are no previous studies aimed at assessing the validity of the screening scales for depression and anxiety in adult patients with bronchiectasis. Accordingly, the purpose of the present study was to analyze the psychometric properties of the self-rating screening scales for depression and anxiety; and to evaluate the concordance for the diagnosis of symptoms of depression and anxiety between these screening scales and the structured clinical interview for DSM-IV in patients with bronchiectasis.
MethodPatientsThis is a cross sectional study that included patients with a diagnosis of bronchiectasis who were monitored periodically (every 2–3 months) in the adult bronchiectasis/cystic fibrosis unit at the Hospital Regional Universitario de Málaga (Málaga, Spain) between May 2012 and May 2013.
Inclusion criteria: patients aged 16 and older with bronchiectasis of any etiology (including cystic fibrosis) who attended the adult bronchiectasis/cystic fibrosis unit for routine review. If at this time they had a respiratory exacerbation or a recent hospital admission, their inclusion was postponed at least 30 days until completion of treatment of the acute process. In all cases bronchiectasis was diagnosed by high-resolution computerized tomography of the chest with the use of a 1–1.5mm window for every 10mm with acquisition times of 1s during full inspiration, following the criteria of Naidich et al.24 All patients underwent a full etiological study following the diagnostic algorithm of bronchiectasis of the Sociedad Española de Neumología y Cirugía Torácica (SEPAR).19 Exclusion criteria: transplant waiting list, problems understanding the questionnaires, absence of written informed consent.
The Research Ethics Committee of the Hospital Regional de Málaga approved the study. All participants gave written informed consent.
MeasurementsThe day of the appointment at the bronchiectasis/cystic fibrosis unit, patients who met inclusion criteria and none of the exclusion criteria were invited to participate. Written informed consent was obtained from each participant before inclusion.
Sociodemographic data were collected, including age, sex, marital/partner status, employment status and educational level.
A full clinical history, from diagnosis through to study participation, was recorded in a regional database. During each visit, clinical variables were collected prospectively, including body mass index (BMI), spirometry and sputum samples for microbiologic analysis. Pulmonary exacerbations were assessed prospectively using the SEPAR criteria.19 Forced vital capacity (FVC) and forced expiratory volume in 1s (FEV1) were expressed in absolute terms (ml) and as a percentage using a reference population.25 The number of exacerbations and hospital admissions in the year prior to the evaluation were also utilized in the analyses. Anxiolytic and antidepressant intake was also recorded.
The day of the inclusion in the study, patients completed the self-reported HADS questionnaire, the BDI, the Hamilton Anxiety Scale and the QOL-B questionnaire (in case of non cystic fibrosis patients) and CFQ-R (in case of cystic fibrosis patients). Afterwards, each participant received a specific appointment with a mental health care professional to conduct a structured clinical interview.
The HADS questionnaire10 comprises two sub-scales, each one ranges from 0 to 21 points; one assesses the symptoms of depression (HADS-D), and the other one the symptoms of anxiety (HADS-A). It was validated in Spanish, including patients with respiratory pathology (chronic obstructive pulmonary disease – COPD).26 The following severity cut-off scores for anxiety and depression have been recommended by the authors of the measure: In each sub-scale, a score below 7 is considered normal range, between 8 and 10 means probable presence of depression or anxiety respectively, and more than 11 suggests the presence of depression or anxiety respectively. These cut-off points have been previously used in patients with Cystic Fibrosis and bronchiectasis.27
The BDI11 is a 21-item-self report-multiple choice inventory. BDI items are rated on a 4-point scale ranging from 0 to 3 based on severity of each item; the maximum total score is 63. The following severity cut-off scores have been recommended: below 9 is considered no depression, between 10 and 18 probable mild depression, between 19 and 29 probable moderate depression, and more than 30 probable severe depression. However, other cut-off points have also been proposed in patients with respiratory diseases such as COPD.28
The Hamilton Anxiety Scale16 consists of 14 items, each defined by a series of symptoms, and measures both psychic anxiety (mental agitation and psychological distress) and somatic anxiety (physical complaints related to anxiety). Each item is scored on a scale of 0 (not present) to 4 (severe), with a total score range of 0–56, where <17 indicates mild severity, 17–24 mild to moderate severity and 25–30 moderate to severe. These cut-off points have been used in patients with COPD.29
The structured clinical interview: patients were individually interviewed by a mental health care professional (who was blinded to the scores obtained in the self-reported questionnaires) using the Spanish version 5.0.0 of the structured Mini International Neuropsychiatric Interview (MINI).30,31 The MINI evaluates for depression and anxiety according to the DSM-IV criteria.9 The outcome in the MINI was considered the gold standard.
QOL-B-Spain: is a disease-specific questionnaire for patients with bronchiectasis in Spanish. It is a self-report measure consisting of 37 questions divided into 8 domains, and takes about 10min to complete. The scores are standardized across 8 scales, ranging from 0 to 100, with higher scores indicating better health-related quality of life.32
CFQR 14+ (Spain): is a disease-specific questionnaire for patients with cystic fibrosis in Spanish. It is a self-report measure consisting of 50 questions and takes about 10–15min to complete. The scores are standardized across 12 scales, ranging from 0 to 100, with higher scores indicating better health-related quality of life.14,33 QOLB and CFQ-R share 8 scales that are the ones analyzed in this work: Physical functioning, Role functioning Vitality, Emotional functioning, Social functioning, Treatment burden, Health perceptions, Respiratory symptoms.
Statistical analysisWe present psychometric properties of the HADS-D, HADS-A, BDI and Hamilton Anxiety Scale at various optimal cut-off values and at traditionally accepted cut-off values. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. Agreement between the screening scales and the diagnoses obtained by the MINI was measured using kappa coefficient and by calculating the area under the receiver operating (ROC) curve (AUC). Maximal discrimination between those with or without a DSM-IV diagnosis was reached at the highest sum of sensitivity and specificity that is termed the Youden index.34 The normality of distributions was verified using the Kolmogorov–Smirnov test. Quantitative variables were compared using t tests or the Mann–Whitney test. Additionally, the Spearman correlation coefficient was employed in order to assess correlations between the screening scales of depression and anxiety, clinical variables and QOL-B and CFQ-R dimensions. To test the statistical significance of the difference between the areas under 2 dependent ROC curves we used the method of DeLong. Confidence levels of 95% were considered in two tail hypothesis tests.
ResultsA total of 52 subjects were included (71.2% women, mean age of 44 years old). Thirty subjects (57.7%) had a diagnosis of non-cystic fibrosis bronchiectasis, and 22 (42.3%) had a diagnosis of cystic fibrosis. Table 1 describes the sociodemographic and clinical characteristics of the study participants.
Sociodemographic and clinical characteristics of the study participants.
| Study variables | N (%) or mean±SDa |
|---|---|
| Age (years) | 44.2±16.2 |
| Sex | |
| Male | 15 (28.8) |
| Female | 37 (71.2) |
| Marital status | |
| Single | 15 (28.8) |
| Married or cohabitant | 33 (63.5) |
| Divorced or separated | 1 (1.9) |
| Widowed | 2 (3.8) |
| Employment status | |
| Able to work | 30 (57.7) |
| Unemployed | 10 (19.2) |
| Retired | 12 (23.1) |
| Educational level | |
| No education | 5 (9.6) |
| Primary school | 19 (36.5) |
| Secondary school | 18 (34.6) |
| College | 10 (19.2) |
| Drug use | |
| Anxiolytics | 12 (23.1) |
| Antidepressants | 5 (9.6) |
| Respiratory disease | |
| Bronchiectasis non-cystic fibrosis | 30 (57.7) |
| Cystic fibrosis | 22 (42.3) |
| FEV1 | |
| Ml | 1971±932 |
| % | 65.8±27.9 |
| Respiratory exacerbations in the last 12 months (n) | |
| Total | 2.02±1.56 |
| Mild | 1.78±1.44 |
| Severe | 0.26±0.53 |
| Colonization of the respiratory tract (n, %) | |
| Any bacteria | 39 (79.6) |
| Haemophilus influenzae | 25 (49.0) |
| Pseudomonas aeruginosa | 36 (70.6) |
| Staphylococcus aureus | 22 (43.1) |
| Methicillin-resistant Staphylococcus aureus | 1 (2.0) |
| Body mass index (kg/m2) | 24.5±5.7 |
| Screening scales | |
| HADS-depression score | 5.8±4.8 |
| HADS-anxiety score | 7.9±5.3 |
| BDI score | 12.1±11.3 |
| Hamilton scale score | 17.1±11.6 |
| MINI | |
| Depression | 18 (34.6) |
| Anxiety | 25 (48.1) |
| Quality of life dimensions | |
| Physical | 56.8±34.0 |
| Role | 70.9±27.7 |
| Vitality | 54.6±27.9 |
| Emotion | 69.1±28.1 |
| Social | 72.2±25.5 |
| Treatment | 66.1±22.6 |
| Health | 46.3±27.4 |
| Respiratory | 73.4±22.5 |
FEV1: forced expiratory volume in the first second; HADS: Hospital Anxiety and Depression Scale.
The results of the MINI based on the DSM-IV criteria showed that 18 subjects (34.6%) had a diagnosis of depression, and 25 subjects (48.1%) had a diagnosis of anxiety.
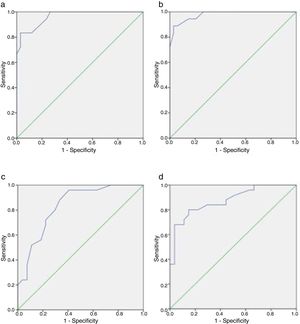
Table 2 summarizes the psychometric properties of the HADS-D subscale and the BDI compared with the MINI for the diagnosis of depression. Optimal cut-off values were ≥9 for the HADS-D (sensitivity 0.833, specificity 0.971), and 17 for BDI (sensitivity 0.889, specificity 0.912). Fig. 1a and b shows the ROC curves for the HADS-D and the BDI, respectively. The AUC was 0.962 (95% CI 0.918–1) for the HADS-D, and 0.978 (95% CI 0.945–1) for the BDI. Difference between areas was 0.0155 (95% CI −0.0378 to 0.0688); p=0.5681.
Psychometric properties at optimal cut-off values and traditionally accepted cut-off values of the depression screening scales.
| Cut-off score | Sensitivity | Specificity | PPV | NPV | Youden index | Kappa | AUC |
|---|---|---|---|---|---|---|---|
| HADS-D | 0.962 (0.918–1) | ||||||
| 6 | 0.944 | 0.765 | 0.680 | 0.963 | 0.709 | 0.650 (<0.001) | |
| 7 | 0.833 | 0.882 | 0.789 | 0.909 | 0.715 | 0.706 (<0.001) | |
| 8 | 0.833 | 0.912 | 0.833 | 0.912 | 0.745 | 0.745 (<0.001) | |
| 9 | 0.833 | 0.971 | 0.937 | 0.917 | 0.804 | 0.826 (<0.001) | |
| 10 | 0.722 | 0.971 | 0.928 | 0.868 | 0.693 | 0.731 (<0.001) | |
| 11 | 0.667 | 1 | 0.100 | 0.850 | 0.667 | 0.723 (<0.001) | |
| BDI | 0.978 (0.945–1) | ||||||
| 10 | 0.944 | 0.794 | 0.708 | 0.964 | 0.738 | 0.685 (<0.001) | |
| 15 | 0.889 | 0.941 | 0.889 | 0.941 | 0.830 | 0.830 (<0.001) | |
| 17 | 0.889 | 0.917 | 0.941 | 0.942 | 0.860 | 0.871 (<0.001) | |
| 18 | 0.833 | 0.971 | 0.937 | 0.917 | 0.804 | 0.826 (<0.001) | |
AUC: area under the curve; BDI: Beck Depression Inventory; HADS-D: Hospital Anxiety and Depression Scale – Depression subscale; NPV: negative predictive value; PPV: positive predictive value. Bold: optimal cut-off values.
HADS-D: AUC 0.962. CI 95% (0.918–1); p<0.0001.
BDI: AUC 0.978. CI 95% (0.945–1); p<0.0001.
ROC curves of the self-rating screening scales compared with MIN. (a) and (b) show the ROC curves for the HADS-D and the BDI, respectively, compared with the MINI for the diagnosis of depression. (c) and (d) show the ROC curves for the HADS-A and the Hamilton Anxiety Scale, respectively, compared with the MINI for the diagnosis of anxiety.
Table 3 summarizes the psychometric properties of the HADS-A subscale and the Hamilton Anxiety Scale compared with the MINI for the diagnosis of anxiety. Optimal cut-off values were ≥4 for the HADS-A (sensitivity 0.960, specificity 0.593) and 17 for the Hamilton Anxiety Scale (sensitivity 0.800, specificity 0.852). Fig. 1c and d shows the ROC curves for the HADS-A and the Hamilton Anxiety Scale, respectively. The AUC was 0.833 (95% CI 0.723–0.943) for the HADS-A, and 0.876 (95% CI 0.781–0.970) for the Hamilton Anxiety Scale. Difference between areas was 0.0422 (95% CI −0.0694 to 0.154); p=0.4585.
Psychometric properties at optimal cut-off values and traditionally accepted cut-off values of the anxiety screening scales.
| Score | Sensitivity | Specificity | PPV | NPV | Youden index | Kappa | AUC |
|---|---|---|---|---|---|---|---|
| HADS-A | 0.833 (0.723–0.943) | ||||||
| 3 | 0.960 | 0.370 | 0.585 | 0.909 | 0.330 | 0.323 (0.004) | |
| 4 | 0.960 | 0.593 | 0.686 | 0.941 | 0.553 | 0.545 (<0.001) | |
| 6 | 0.880 | 0.667 | 0.710 | 0.857 | 0.547 | 0.542 (<0.001) | |
| 7 | 0.800 | 0.704 | 0.714 | 0.792 | 0.504 | 0.501 (<0.001) | |
| 11 | 0.560 | 0.815 | 0.737 | 0.667 | 0.375 | 0.378 (0.005) | |
| Hamilton | 0.876 (0.781–0.970) | ||||||
| 6 | 0.960 | 0.333 | 0.500 | 0.900 | 0.293 | 0.286 (0.007) | |
| 15 | 0.840 | 0.704 | 0.724 | 0.826 | 0.544 | 0.541 (<0.001) | |
| 16 | 0.800 | 0.778 | 0.769 | 0.808 | 0.578 | 0.577 (<0.001) | |
| 17 | 0.800 | 0.852 | 0.833 | 0.821 | 0.652 | 0.653 (<0.001) | |
| 18 | 0.760 | 0.852 | 0.826 | 0.793 | 0.612 | 0.614 (<0.001) | |
AUC: area under the curve; HADS-A: Hospital Anxiety and Depression Scale – Anxiety subscale; NPV: negative predictive value; PPV: positive predictive value.
Bold: optimal cut-off values in bold.
HADS-A: AUC 0.833. CI 95% (0.723–0.943); p<0.0001.
Hamilton: AUC 0.876. CI 95% (0.781–0.970); p<0.0001.
We found significant correlations between the score of the scales HADS-D, HADS-A, BDI and the Hamilton Anxiety Scale with each other and with all the dimensions of the QOL-B and CFQ-R, except for treatment burden. We do not observe significant correlations of the scores of the scales with clinical variables such as BMI, age, FEV1%, or exacerbations rate (Table 4). Patients with Pseudomonas aeruginosa or any chronic bronchial infection did not have significantly different scores from any of the self-rated screening scale for depression and anxiety studied relative to those not colonized (data not shown).
Correlations (Spearman correlation coefficient) between the screening scales of depression and anxiety, clinical variables and quality of life score.
| Correlation coefficient | HADS-A | HADS-D | BDI | Hamilton |
|---|---|---|---|---|
| HADS-A | 1.000 | .763*** | .782*** | .702*** |
| HADS-D | .763*** | 1.000 | .838*** | .772*** |
| BDI | .782*** | .838*** | 1.000 | .810*** |
| Hamilton | .702*** | .772*** | .810*** | 1.000 |
| BMI | .000 | .103 | .089 | −.014 |
| Age | .061 | .225 | .113 | .212 |
| FEV1% | −.026 | −.149 | .002 | −.044 |
| Exacerbation | .081 | −.039 | −.060 | −.101 |
| QOL Physical | −.559*** | −.633*** | −.520*** | −.511*** |
| QOL Role | −.588*** | −.673*** | −.676*** | −.521*** |
| QOL Vitality | −.748*** | −.711*** | −.767*** | −.694*** |
| QOL Emotion | −.748*** | −.711*** | −.767*** | −.694*** |
| QOL Social | −.488*** | −.465** | −.437** | −.315* |
| QOL Treatment | −.244 | −.267 | −.295 | −.156 |
| QOL Health | −.685*** | −.609*** | −.701*** | −.589*** |
| QOL Respiratory | −.445** | −.482** | −.536*** | −.471** |
BDI: Beck Depression Inventory; HADS: Hospital Anxiety and Depression Scale; HADS-A: Hospital Anxiety and Depression Scale – Anxiety subscale; HADS-D: Hospital Anxiety and Depression Scale – Depression subscale; QOL: quality of life score.
Spearman correlation coefficient.
Anxiety and depression are commonly reported in patients with bronchiectasis, therefore in this group of patients it is especially important to screen for the presence of symptoms of these psychiatric disorders in the Bronchiectasis Units, and refer the right patients to a mental health team for treatment. The prevalence varies among different studies, which depends on the method of diagnosis. In previous studies performed in this group of patients using screening scales to diagnose depression and anxiety, the prevalence of depression symptoms ranges between 20 and 34%, and the prevalence of anxiety between 38 and 55%.20–23 In the present study, the MINI revealed a prevalence of depression of 34.6% and anxiety of 48.1%. To our knowledge this is the first study in patients with bronchiectasis in which a structured clinical interview was performed.
The optimal cut-off values of the screening scales in patients with bronchiectasis might differ from the ones traditionally accepted, as have been reported previously in other diseases. Optimal cut-off values for the diagnosis of depression using HADS-D ranges between 7 in patients on hemodialysis35 and in patients with obstructive pulmonary disease,28 and 8 in patients with coronary artery disease13 and in patients with systemic lupus erythematosus.36 For the BDI, an optimal cut-off point of 14 was reported in a study with patients on hemodialysis,35 and an optimal cut-off point of 13 was found in patients with obstructive pulmonary disease.28 For the anxiety screening scales, the optimal cut-off point for HADS-A was 6 in patients with systemic lupus erythematosus36 and in patients on hemodialysis,35 8 in patients with coronary artery disease,13 9 in patients with obstructive pulmonary disease.28
However, we did not find any studies that assess the psychometric properties of the screening scales to detect depression and anxiety in patients with bronchiectasis. We believe it to be of interest because of the high prevalence of these psychiatric disorders in this specific group of patients, and the negative consequences of the under-diagnosis and under-treatment of these psychiatric symptoms on the quality of life and disease severity.20–22
Therefore, in the present study we assessed the optimal cut-off points of the screening scales (HADS-D, BDI, HADS-A and Hamilton Anxiety Scale) to determine the most sensitive and specific value to detect depression and anxiety of each scale in this specific group of patients. To detect depression, we found that the optimal cut-off value for the HADS-D was 9, and for the BDI it was 14. These cut-off points for both scales are close to, but are not the standard recommendation.10,11 Additionally, both HADS-D and BDI have a high and similar AUC to detect depression and may be used indistinctly as a tool for screening depression in patients with bronchiectasis. To detect anxiety, our results showed that the optimal cut-off value for HADS-A was 4, and for the Hamilton Anxiety Scale was 17. In this case, we found that the standard cut-off point recommendation for HADS-A has a low sensitivity in patients with bronchiectasis, thus we propose using 4 as the cut-off point in order to improve the performance of the HADS-A in this group of patients. For the Hamilton Anxiety Scale, we suggest using 17 as a cut-off point to detect anxiety. HADS-A and the Hamilton Anxiety Scale have a good and similar AUC to detect anxiety.
The scores of the three scales evaluated (HADS, BDI and Hamilton) have presented significant negative correlations (higher scores, worse quality of life) with the 8 dimensions of the patients reported outcome QOL-B and CFQ-R evaluated, except for treatment burden, being especially high in the emotional functioning dimension. On the contrary, we do not observe significant associations of HADS, BDI or Hamilton scores, with individual important components such as BMI, age, P. aeruginosa or any chronic bronchial infection, FEV1%, or exacerbations rate. These results are similar to those previously found by our group where symptoms of depression and anxiety were significant predictors of health-related quality of life in patients with bronchiectasis, independently of respiratory involvement, age or other variables. These results reinforce the importance of assessing symptoms of depression/anxiety in bronchiectasis because it adds new information on the impact of the disease on the patient. So the current multidimensional scoring systems like such as the Bronchiectasis Severity Index (BSI), FACED, and e-FACED37–39 developed in bronchiectasis are useful to assess the clinical severity and prognosis of the disease, but they do not include other important dimensions such us the impact of bronchiectasis upon the patient.40,41
The strength of the study is that is the first paper on this topic in patients with bronchiectasis and includes the evaluation of associations of screening test with clinical and quality of life variables. The limitation is that we have included patients with and without CF (and probably the characteristics of these two groups could be different). In any case, a specific test for CF such as CFQ-R and non-bronchiectasis for QOL-B has been used for the evaluation of quality of life, since both questionnaires share the same 8 dimensions analyzed in this study.
In conclusion, the self-rating screening scales HADS, BDI and Hamilton Anxiety Scale are reliable tools to screen for depression and anxiety in adult patients with bronchiectasis. However, our results suggest that the use of specific cut-off values may improve the diagnostic accuracy of the previous scales in patients with bronchiectasis.
Authors’ contributionsG.O. and C.O. substantially contributed to the conception and design of the study, acquisition, analysis, and interpretation of the data; statistical analysis; and drafting of the manuscript. N.C. contributed to the acquisition, analysis, and interpretation of the data; statistical analysis; revised the article for important intellectual content and drafting of the manuscript; J.H.-P., T.B., J.F.-R., N.P. M.V.G., and L.F.D.R. contributed to the data acquisition and critical review of the manuscript.
G.O. is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interestsThe authors declare no conflict of interest.
We would like to thank every patient has participated in this study.