SARS-CoV-2 disease (COVID-19) is an infection caused by a new emerging coronavirus, first detected in Wuhan, China, in December 2019. It has now become a pandemic and is posing a serious public health problem for almost all countries.1 Typical radiological manifestations in patients with SARS-CoV-2 pneumonia consist of the presence of bilateral pulmonary opacities (both ground glass attenuations and consolidations) with a peripheral/subpleural distribution, often involving the posterior regions of both lungs specifically.2,3 A recent series of patients with COVID-19 indicated that 1% of patients may develop pneumothorax as a complication.4 Some sporadic cases of spontaneous pneumothorax and/or pneumomediastinum have been reported in patients with COVID-19 with varying outcomes, and it remains unclear whether these events are a potential indicator of worsening infection.5–8 In this paper, we describe 4 cases of spontaneous pneumomediastinum (SP) in patients with COVID-19.
These were 4 patients (58–65 years of age, 2 men and 2 women) who attended two hospitals in Madrid with fever and/or chest symptoms (cough, dyspnea and/or pain) during the months of March and April 2020, coinciding with the peak of the SARS-CoV-2 pandemic (COVID-19) that was ravaging Spain and, in particular, the Madrid region at the time. Tests for the detection of SARS-CoV-2 nucleic acid by polymerase chain reaction (RT-PCR) and a chest X-ray were performed in the emergency department in all 4 patients. RT-PCR was positive in all patients, and chest X-ray showed (also in all 4 cases) bilateral opacities suggesting infection.
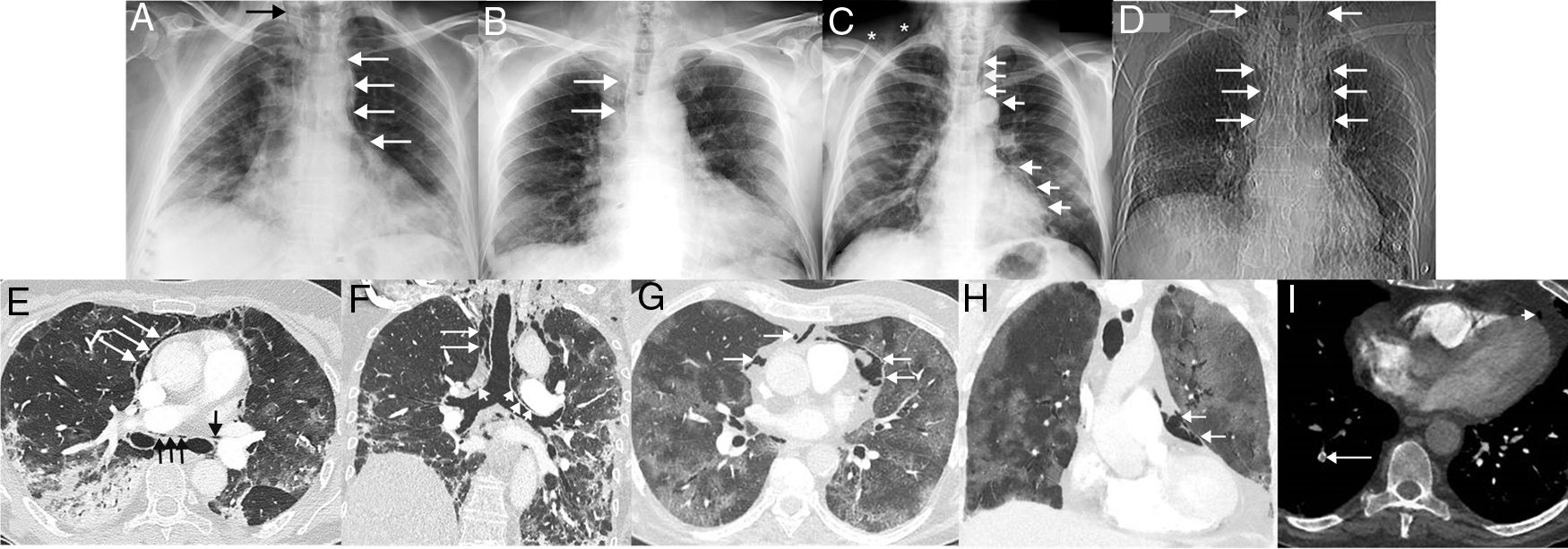
Once admitted, treatment of SARS-CoV-2 infection started and other drugs (antipyretics, bronchodilators, corticosteroids, etc.) were used depending on the particular needs of each patient. All 4 patients required oxygen administration via nasal prongs or masks (simple or reservoir) during admission (before the onset of SP), but did not require mechanical ventilation. None of the four patients had a history of smoking and none had a previous history of pneumothorax, SP or lung disease. During admission, the course of the 4 patients was complicated by SP (unrelated to invasive procedures such as tracheal intubation or tracheotomy), which was not clinically suspected in any case, and first detected on chest X-ray in 3 patients and on computed tomography (CT) in 1 case in which pulmonary thromboembolism was suspected. Chest X-rays identified the presence of ectopic gas dissecting the tissues of the mediastinum and the neck (Fig. 1A–D). In 1 of the patients, CT revealed consolidations, traction bronchiectasis and the presence of gas dissecting the mediastinum, including the walls of both main bronchi, were observed (Fig. 1E–F). In another patient, CT revealed extensive crazy-paving opacities (association of ground glass attenuation opacities and pulmonary interstitial thickening) and a small amount of gas in the prevascular mediastinal fat (Fig. 1G–H). The clinical course was favorable in 3 of the 4 cases; 1 patient died from infectious complications unrelated to SP (Pseudomonas aeruginosa pneumonia and P. aeruginosa and Enterococcus faecalis bacteremia). One of the patients with favorable progress was diagnosed with pulmonary thromboembolism on chest angio-CT (Fig. 1I). No SP required surgical treatment and all 4 cases were managed conservatively, with SP disappearing in radiological controls. Table 1 summarizes the most important clinical aspects of the 4 patients with SP.
(A) Posteroanterior chest X-ray in a 60-year-old woman admitted with bilateral SARS-CoV-2 pneumonia showing incidental pneumomediastinum (white arrows). Note the spread of gas to the soft tissue of the neck (black arrow). (B) Posteroanterior chest X-ray in a 62-year-old man admitted with bilateral SARS-CoV-2 pneumonia, who presented chest pain and dyspnea. Right paratracheal pneumomediastinum (arrows) is seen on the X-ray. (C) Posteroanterior chest X-ray in a 58-year-old man with bilateral SARS-CoV-2 pneumonia, who had an episode of chest pain and hypotension. On the X-ray, pneumomediastinum with pneumopericardium (arrow) and gas is seen in the soft tissue of the right supraclavicular region (asterisks). (D) Topogram (corresponding to a chest CT scan) of the only CT-diagnosed pneumomediastinum in a 64-year-old woman with bilateral SARS-CoV-2 pneumonia, who presented with chest pain and dyspnea. The topogram shows a large pneumomediastinum extending to the soft tissue of the neck (arrows). (E) Axial CT image of the chest (lung window) of the patient in image D confirming the presence of ectopic gas surrounding the main bronchi (black arrows) and dissecting the pericardium (white arrows). (F) Coronal chest CT image (lung window) of patient in image D in which air can be seen dissecting both main bronchi (short arrows) and right paratracheal fatty tissue (long arrows). (G) Axial chest CT image (lung window) of patient in image B confirming the presence of gas dissecting the pericardium (arrows). H) Coronal chest CT image (lung window) of the patient in image B showing gas dissecting the pericardium (arrows). (I) Axial CT image of the chest (mediastinum window) of the patient in image B, identifying a defect in a subsegmental artery in the right lower lobe (long arrow). Note the presence of gas in pericardial fat (short arrow).
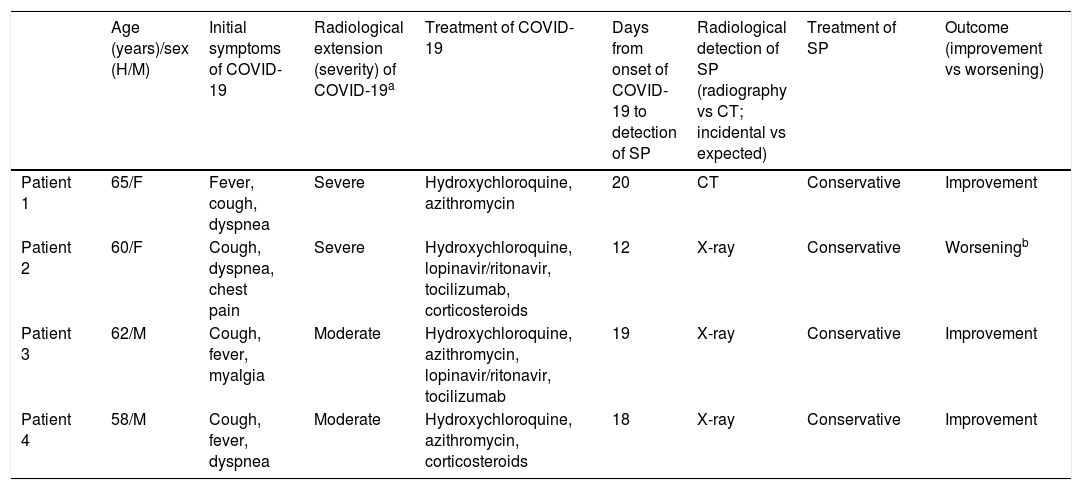
Clinical characteristics of patients with COVID-19 and SP.
| Age (years)/sex (H/M) | Initial symptoms of COVID-19 | Radiological extension (severity) of COVID-19a | Treatment of COVID-19 | Days from onset of COVID-19 to detection of SP | Radiological detection of SP (radiography vs CT; incidental vs expected) | Treatment of SP | Outcome (improvement vs worsening) | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 65/F | Fever, cough, dyspnea | Severe | Hydroxychloroquine, azithromycin | 20 | CT | Conservative | Improvement |
| Patient 2 | 60/F | Cough, dyspnea, chest pain | Severe | Hydroxychloroquine, lopinavir/ritonavir, tocilizumab, corticosteroids | 12 | X-ray | Conservative | Worseningb |
| Patient 3 | 62/M | Cough, fever, myalgia | Moderate | Hydroxychloroquine, azithromycin, lopinavir/ritonavir, tocilizumab | 19 | X-ray | Conservative | Improvement |
| Patient 4 | 58/M | Cough, fever, dyspnea | Moderate | Hydroxychloroquine, azithromycin, corticosteroids | 18 | X-ray | Conservative | Improvement |
CT: computed tomography; F: female; M: male; SP: spontaneous pneumomediastinum.
One of the plausible mechanisms by which SP can occur in patients with COVID-19 is the diffuse alveolar damage that occurs in any severe pneumonia. Three of the 4 patients in our series had extensive bilateral pneumonia (involving all lobes in both lungs), while the other had less severe but also bilateral disease. According to a published score for quantifying the extent of SARS-CoV-2 pneumonia, 2 patients were “severe” and the other 2 were “moderate”.9 All 4 patients had intense repetitive episodes of dry cough; these episodes of cough are known to produce a sudden increase in distal airway pressure, causing alveolar rupture and secondary gas leakage to the peribronchovascular pulmonary interstitium, from where the air can dissect proximally, finally reaching the mediastinum. This phenomenon, called the “Macklin effect”, has been implicated as the cause of pneumomediastinum that appears in some closed thoracic lesions, asthma attacks, and Valsalva maneuvers.10,11 In some cases of pneumomediastinum caused by infectious processes, CT images of subpleural bullae or cysts have been described, but in the 2 patients in our series who underwent CT, no bullae, cysts, pulmonary emphysema or pneumothorax were detected. Pneumomediastinum (and pneumothorax) is a relatively common complication in patients undergoing mechanical ventilation, but our 4 cases had only received oxygen therapy via nasal prongs or masks prior to SP. Moreover, the patient with unfavorable progress required tracheal intubation 3 days after radiographic detection of SP, but on the first X-ray after intubation the pneumomediastinum was no longer observed. SP is a very rare complication of viral pneumonia. Some isolated cases associated with SARS pneumonia (severe acute respiratory syndrome), influenza A pneumonia (H1N1), and SARS-CoV-2 pneumonia have been published.5–8,12,13 No other possible causes of pneumomediastinum were detected in any the 4 patients in our series, for which reason we believe that they were caused by SARS-CoV-2 infection.
The exact mechanism by which SP occurs in SARS-CoV-2 pneumonia is unknown, and while SP is in principle considered a self-limiting condition that responds favorably to conservative therapeutic measures, the progress of these patients should be monitored for the possibility of pneumomediastinum-related cardiovascular and respiratory complications.14 Although this paper describes the largest series to date of SP in patients with COVID-19, more cases must be studied to determine its prognostic significance and, if it is identified as a marker of disease progression, specific therapeutic measures and recommendations must be established.
Conflict of interestThe authors state that they have no conflict of interest.
Please cite this article as: Gorospe L, Ayala-Carbonero A, Ureña-Vacas A, Fra Fernández S, Muñoz-Molina GM, Arrieta P, et al. Neumomediastino espontáneo en pacientes con COVID-19: una serie de cuatro casos. Arch Bronconeumol. 2020;56:754–756.