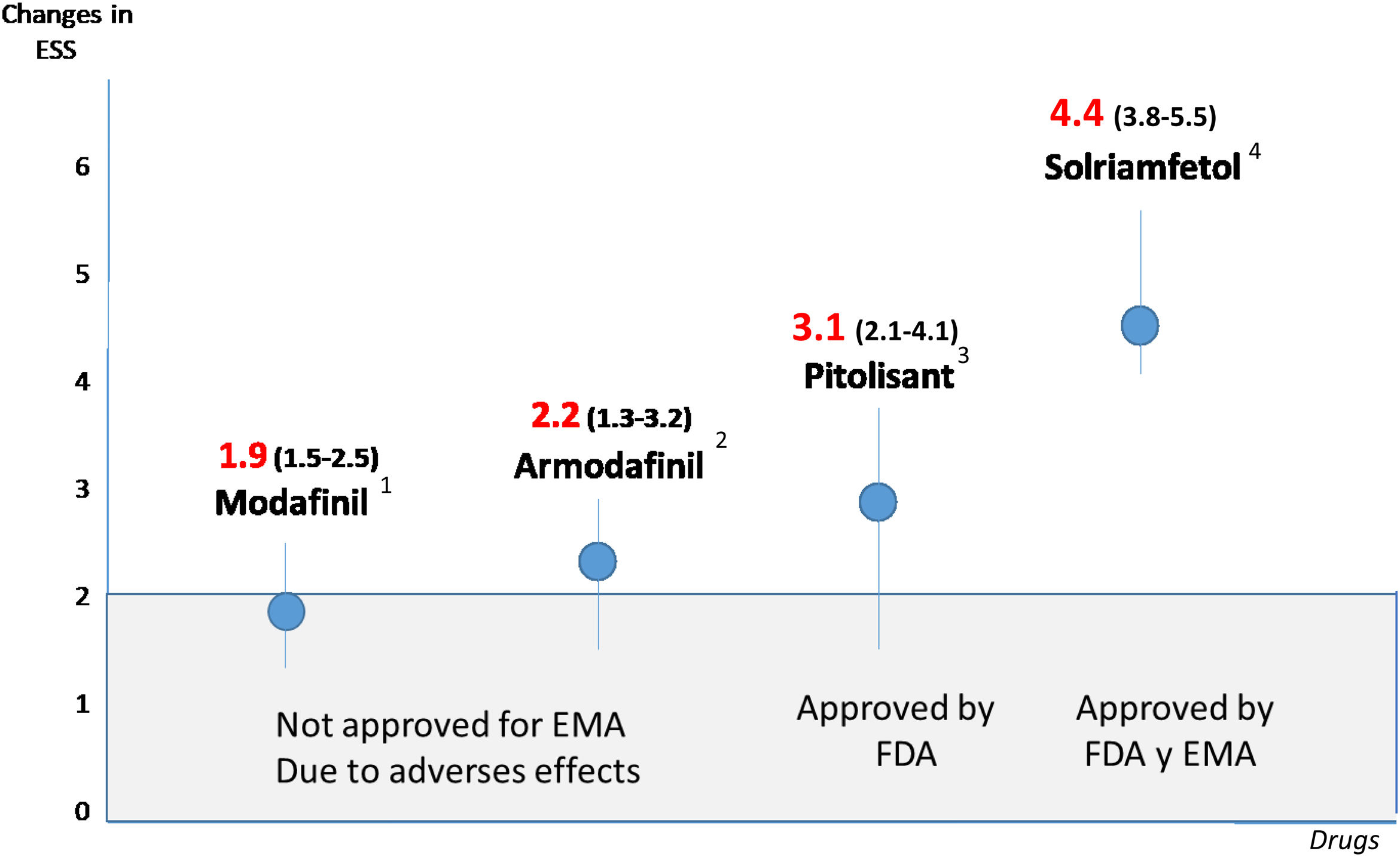
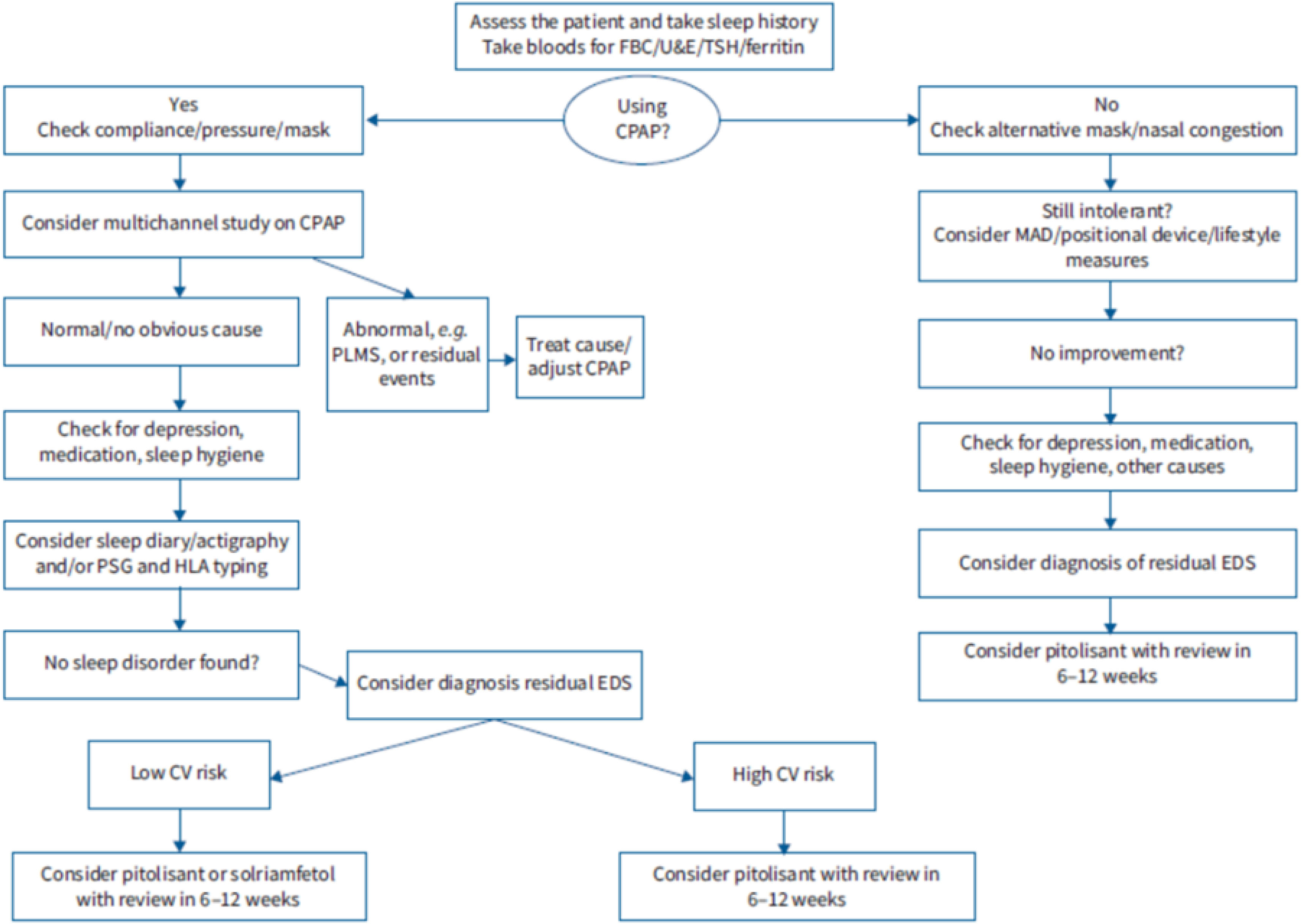
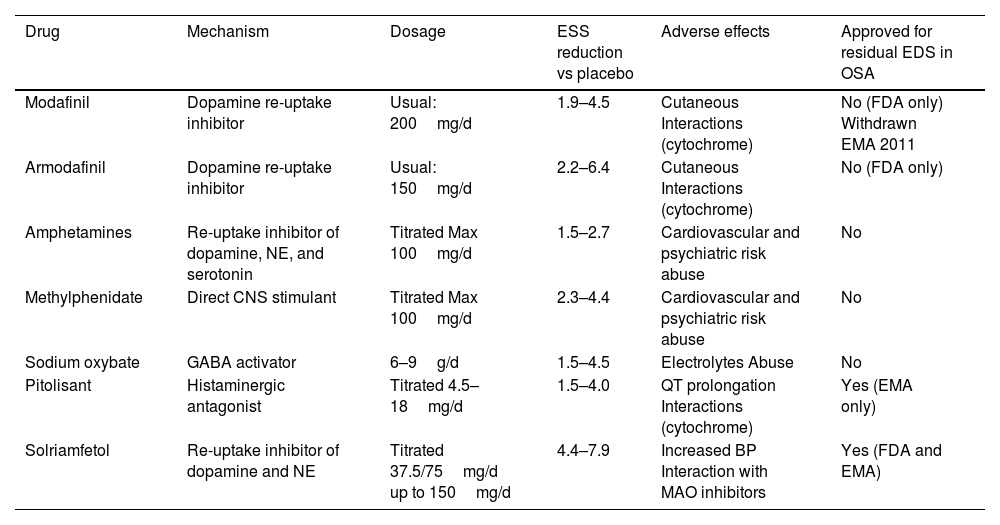
Excessive daytime sleepiness is one of the most important symptoms in obstructive sleep apnoea and it is often used to decide whether the latter should be diagnosed as a syndrome or considered a priority in diagnosis and treatment, and whether the treatment is effective. Beyond this, one concept of enormous clinical significance has emerged: “residual excessive daytime sleepiness”, defined as excessive daytime sleepiness in the context of a diagnosed obstructive sleep apnea that is treatable with continuous positive airway pressure (CPAP) or alternatives to it; it appears as a consequence of poor adaptation to, or refusal of treatment, or even sometimes in situations of good tolerance. Given the direct relationship between excessive daytime sleepiness (usually defined by an Epworth Sleepiness Scale [ESS] value of more than 10 points) and cardiovascular, neurocognitive and quality-of-life disorders, attempts have been made for years to alleviate it with drugs (modafinil, armodafinil, sodium oxybate or amphetamines), to little effect and with a large number of adverse effects. However, unlike their predecessors, two products that have recently appeared on the market – solriamfetol and pitolisant – have achieved clinically significant reductions without any major adverse effects, in most cases. In fact, a Task Force from the European Respiratory Society has even proposed an algorithm for residual excessive daytime sleepiness and its pharmacological treatment in the context of obstructive sleep apnoea. The present review aims to define the importance of excessive daytime sleepiness, especially residual excessive daytime sleepiness in patients with obstructive sleep apnoea, as well as the old and new pharmacological alternatives that have appeared.
The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years.
© Clarivate Analytics, Journal Citation Reports 2025
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact.
See moreSNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field.
See more