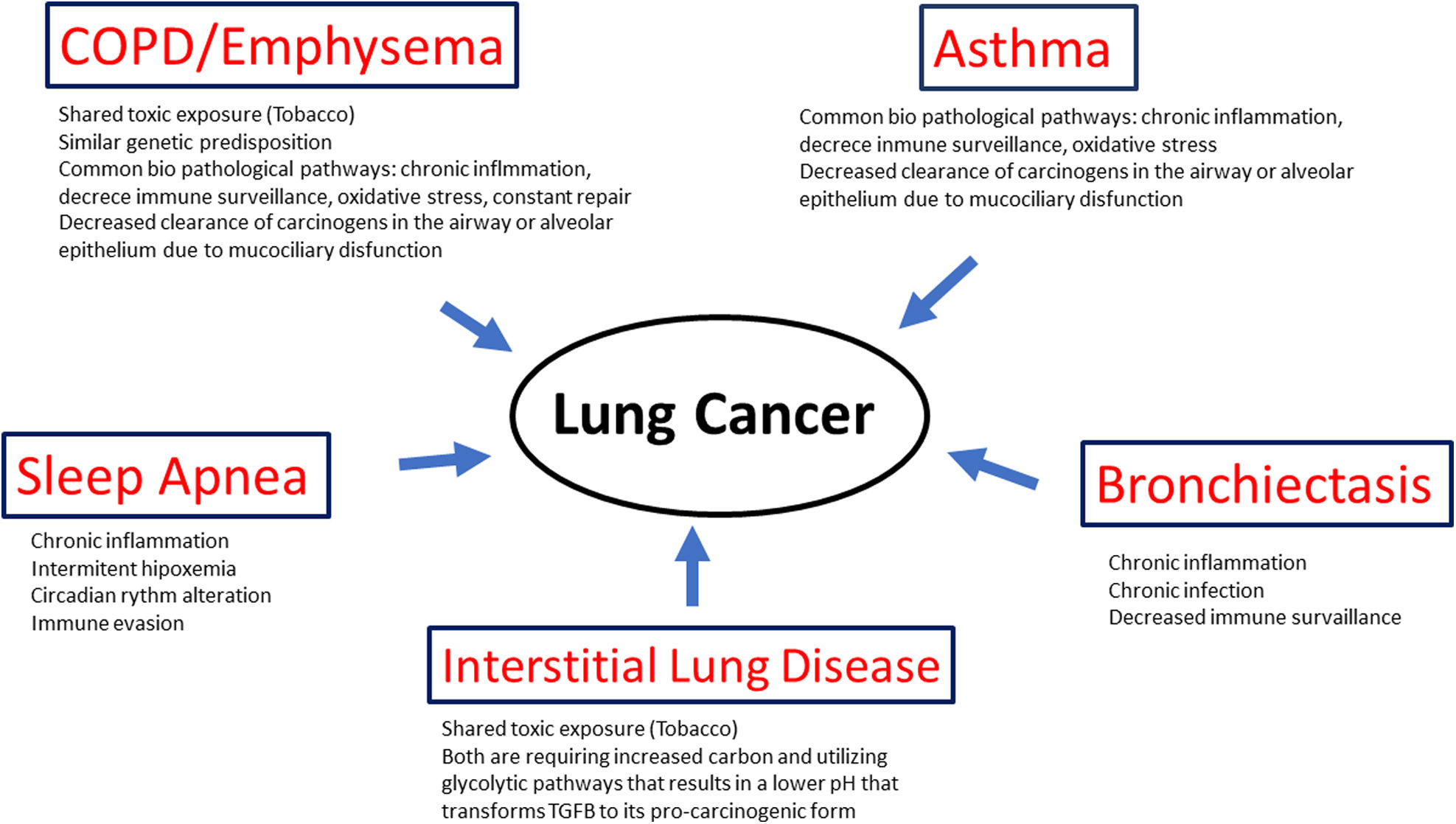
Lung cancer (LC), the leading cause of death due to malignancy in both males and females, is common in patients with coexisting respiratory comorbidities. Many of these share common etiologies with LC such as smoking, biomass fume or occupational exposure, and ambient air pollution, but also a genetic predisposition and/or common pathophysiologic mechanisms, chief among them chronic inflammation, altered immune surveillance, cell injury and increased turnover, to name a few. This common thread puts patients with respiratory comorbidities at increased risk of developing LC.
The present article reviews why patients with 5 of the most prevalent chronic respiratory diseases (COPD, asthma, Interstitial Lung Disease, Obstructive Sleep Apnea and Bronchiectasis) are at increased risk of LC and the potential pathological mechanisms underlying this clinically relevant association.
Lung cancer (LC), the leading cause of cancer deaths worldwide, usually appears in the context of some of the most prevalent chronic respiratory diseases including chronic obstructive pulmonary disease (COPD), especially the emphysema phenotype, asthma, interstitial lung disease, obstructive sleep apnea (OSA) and bronchiectasis (BE). The epidemiologic association is strong enough that most of them are considered independent risk factors for LC. Inhalation of toxic tobacco smoke, fine particulate matter or occupational exposure in the context of a favorable genetic milieu, and a shared pathophysiology have been postulated to explain this deadly association. Hypoxemia and chronic inflammation, as well as altered immune surveillance have also been proposed as potential partners in causality.
The present brief review by experts in each chronic respiratory disease summarizes the evidence behind these association and the potential biological mechanisms that provide the common thread linking seemingly unrelated respiratory comorbidities and LC.
Chronic obstructive pulmonary disease and lung cancerCOPD is defined by the Global Initiative for Obstructive Lung Disease (GOLD) Guidelines [1], as the presence of airflow obstruction (AO) (forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) ratio of <0.70) in patients with risk factors (mainly tobacco smoking) and symptoms of the disease. Although emphysema (a permanent enlargement of air spaces distal to the terminal bronchioles, accompanied by the destruction of alveolar walls and without obvious fibrosis) often co exists in most of patients with COPD (up to 70%), they are different entities, both with an independent increased risk of developing LC.
An association between AO and LC was described almost 40 years ago by Skillrud et al. [1] and Tockman et al. [2]. A meta-analysis by Wasswa-Kintu et al. [3] showed that even a modest reduction in airflow significantly predicts LC risk. A thoughtful and prescient editorial by Petty [4] suggested in 2005 that COPD and LC could have a common etiology based on shared inflammatory processes, genetic predisposition and environmental risk factors. LC, emphysema, and AO are among the deadliest of preventable diseases and are all caused or aggravated by smoking. While the causal link with tobacco is obvious, it wasn’t until the widespread use of low dose CT to screen for LC that the preeminent role of the emphysema phenotype became apparent. In near simultaneous publications de Torres et al. [5] from Spain, and Wilson et al. [6] from the United States, demonstrated that patients with emphysema enrolled in a LC screening program, had a three to four-fold increased incidence of LC. Similar results have been reported in other screening cohorts and in a recently published meta-analysis, in which the odds ratio (OR) for LC in individuals with CT detected emphysema was 2.45 (1.85–3.05) [7]. Fortunately, the studies that analyzed the association of AO and emphysema were properly designed, performed in different populations and adjusted for the most important cofounding factors (age, smoking status, smoking history, sex, AO itself) suggesting that the quality and the strength of the evidence is robust.
COPD and emphysema are both independently associated with an increased risk of developing LC, probably due to the inflammation that is the hallmark of these entities. There are several possible explanations that justify an association between AO present in COPD, emphysema, and LC. The most intuitive and timely is a shared causal pathway from lung and airway inflammation [8]. Tobacco smoke is well known to stimulate inflammation [9] and chronic inflammation has been suggested as an important etiologic factor in COPD [10] and emphysema [11]. In fact, small airways obstruction has been described in both emphysema and COPD with the thickness of the walls of the small airways being closely associated with the severity of COPD [10] and emphysema [11]. Chronic inflammation has also been implicated in the pathogenesis of many cancers, including LC [12,13]. It is plausible that chronic inflammation in the airways, a hallmark in the pathogenesis of both emphysema and COPD, may result in repeated injury and subsequent repair, stimulating cell turnover and cumulative genetic errors, which may ultimately lead to oncogenesis and field cancerization [14]. A study of bronchoalveolar stem cells in a murine model provides further support for a shared pathogenesis [15]. Bronchoalveolar stem cells exhibit proliferative capacity and self-renewal, in response to both lung injury and oncogenic K-ras stimulation. The fact that the presence of any degree of emphysema, more so than the severity of emphysema, is associated with LC is consistent with a common pathogenesis for lung injury, attempted repair and tumor proliferation.
The association between emphysema and LC is stronger than the association between the latter and AO, although both contribute to an increased risk [16]. LC is a leading cause of death in patients with COPD. Not surprisingly, the two most important interventions capable of reducing deaths from LC include primary prevention with smoking cessation, and secondary prevention with the widespread implementation of LC screening programs. Potential future lines of research include the role of AO and emphysema and the tumor microenvironment, host–microbiome interactions in COPD and LC [17] and shared molecular pathways including the mutational profile and burden of LCs arising in the context of COPD and emphysema.
Asthma and lung cancerThe potential role of asthma as an independent risk factor for LC has been evaluated in different types of studies with mixed findings and questionable quality to support the association. Early studies evaluating the association between asthma and LC showed an inverse relationship, suggesting that asthma may have a protective effect by stimulating immune surveillance [18,19]. More recently, emerging evidence based on multiple case-control and cohort studies have found that asthma may be a modest risk factor for LC in both smokers and non-smokers [20–22].
A 2003 meta-analysis including data from 7 case-control and 4 cohort studies showed that patients with asthma had a significant increase in LC risk after adjusting for smoking history (relative risk [RR]: 1.6, 95% confidence interval [CI]: 1.3–1.9) (Table 1) [23]. Interestingly, a subset analysis limited to 5 case-control studies including never smokers also showed an increased risk of LC among patients with asthma (RR: 1.8, 95% CI: 1.3–2.3], indicating that asthma itself poses a risk for LC independent of tobacco exposure. One of the largest systematic reviews, a pooled analysis from the International LC Consortium including more than 585,000 participants, reported an overall relative risk of about 1.28 (95% CI: 1.16–1.41) for LC in individuals with asthma. Both squamous cell (RR: 1.69, 95% CI: 1.26–2.26) and small cell tumors appeared to be more frequent in patients with asthma (RR: 1.71, 95% CI: 0.99–2.95) but not adenocarcinoma (RR: 1.09, 95% CI: 0.88–1.36 [24]. Notably, the excess LC incidence was highest within the first 2 years following the diagnosis of asthma and diminished over time. Finally, a recent Mendelian randomization analysis demonstrated that genetic susceptibility to asthma is associated with increased LC risk (odds ratio: 1.11, 95% CI: 1.04–1.17), supporting a causal relationship [25]. It seems that the quality and the strength of available evidence is acceptable (performed in different populations and adjusting for important cofounding such age, sex, smoking, etc.) indicating that asthma is an independent risk factor for LC, albeit with a relatively modest effect size (on the order of a 1.2–1.5-fold RR).
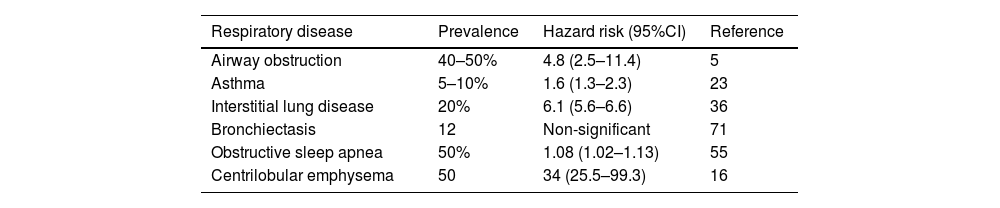
Prevalence and hazard ratio of LC development of each chronic respiratory disease.
| Respiratory disease | Prevalence | Hazard risk (95%CI) | Reference |
|---|---|---|---|
| Airway obstruction | 40–50% | 4.8 (2.5–11.4) | 5 |
| Asthma | 5–10% | 1.6 (1.3–2.3) | 23 |
| Interstitial lung disease | 20% | 6.1 (5.6–6.6) | 36 |
| Bronchiectasis | 12 | Non-significant | 71 |
| Obstructive sleep apnea | 50% | 1.08 (1.02–1.13) | 55 |
| Centrilobular emphysema | 50 | 34 (25.5–99.3) | 16 |
Several pathophysiologic mechanisms could explain the association between asthma and LC. Asthma causes chronic inflammation including the release of cytokines, growth factors, and reactive oxygen species, along with repeated epithelial injury [26,27] inducing DNA damage and aberrant cell proliferation, thereby promoting oncogenesis. This paradigm is analogous to other chronic lung diseases, such as the aforementioned COPD, where long-standing inflammation predisposes to LC. Immune dysregulation in asthma may also play a role. Asthma is typically associated with a T-helper type 2-immune response and altered local immunity, which might impair anti-tumor surveillance. Asthma may also increase LC risk by impaired clearance of carcinogens in the airway or alveolar epithelium due to muco-ciliary dysfunction [28]. Finally, treatment with inhaled corticosteroids, which has been associated with a decreased risk of LC in patients with COPD [29] may also play a chemo preventive role in asthmatics [30].
Interstitial lung disease and lung cancerPulmonary fibrosis causes scarring of the lung parenchyma with associated volume loss and architectural distortion. There are two pharmaceutical treatments approved to slow its progression [31]. Initially these new treatments were only indicated for Idiopathic Pulmonary Fibrosis (IPF), but their use has been expanded to include progressive fibrosis allowing many more people to benefit from antifibrotic therapy [32]. The incidence of pulmonary fibrosis is increasing globally. Fortunately, mortality has decreased with current antifibrotic treatments [33]. Fisher demonstrated that patients treated with pirfenidone regained 25% of their life expectancy which would otherwise be lost due to fibrosis [34].
The longer a patient lives with pulmonary fibrosis the greater the risk for developing LC. In a multicenter European study, Karampitsakos found that 10 years after being diagnosed with IPF 27% of patients who were still alive had developed LC [35]. In a large Korean study of over 343,000 people, approximately 4300 developed LCs, including 5% of those with COPD, 6% of those with fibrosis, and 12% of those with the combined emphysema fibrosis phenotype [36]. Based on that study, the combination of fibrosis and emphysema appears to carry a higher risk of developing LC than fibrosis alone or emphysema alone. Acknowledging that there are only a few long-term observational studies that explored the association between ILD and LC, most of them were well designed, in large populations from different settings and adjusted for the most important cofounding factors, giving them enough quality and strength to support that association.
Pulmonary fibrosis meets the National Cancer Institute's 5 criteria for a premalignant condition which include; high likelihood of cancer, cancer develops from the precancer, the precancer is distinct from the native tissue, the condition has characteristics of cancer and the condition can be diagnosed with various techniques. Therefore, it is not surprising that autopsies frequently reveal that fibrotic lung harbor LC, coinciding in up to 48% of deceased individuals in one study [37,38]. The growing periphery of a LC is analogous to the leading edge of pulmonary fibrosis in that both are requiring increased carbon and utilizing glycolytic pathways that results in a lower pH that transforms TGFB to its pro-carcinogenic form [39].
LC occurring in the setting of fibrosis differ from cancers occurring in non-fibrotic lungs [40,41]. Salvatore et al. reviewed 4434 chest CT scans of patients with reported fibrosis. Many patients (609) had pulmonary nodules, including 274 in the fibrotic lung and 335 nodules in the nonfibrotic parenchyma [40]. Of the 274 nodules in fibrosis, 93 were cancer and of the 335 nodules seen in non-fibrotic lung only 50 were cancer. Therefore, a nodule embedded in fibrotic lung tissue is more likely to be cancer than a nodule appearing in the normal lung parenchyma. The most common cell type for LC in nonfibrotic lung is adenocarcinoma, while 14% are squamous cell cancers [41]. However, 32% of cancers in fibrotic lungs had a squamous cell histology and faster doubling times. Furthermore, small lung nodules in normal lung parenchyma measuring less than 6mm are rarely malignant (<1%), while as many as 10% of similar lung nodules in the fibrotic lung [41]. Cancer occurring in fibrosis behaves more aggressively perhaps because the paucity of vessels in that microenvironment impairs immune surveillance [42,43]. Treatment of cancers occurring in pulmonary fibrosis is challenging with increased likelihood of a pulmonary exacerbation and associated mortality [44].
Smoking is a known risk factor for the development of pulmonary fibrosis. A minority of individuals who meet criteria for LC screening have evidence of pulmonary fibrosis on CT (6.6%), so LC screening is an ideal way to diagnose pulmonary fibrosis early, implement smoking cessation and treatment early in order to prevent progression and continue screening for LC [45]. Usual interstitial pneumonia (UIP) is an independent LC risk factor and therefore patients with pulmonary fibrosis should be screened for LC annually [46]. Furthermore, screening guidelines for patients with emphysema may need to be modified for patients with pulmonary fibrosis as the likelihood of cancer is higher even for smaller nodules while tumor aggressiveness is greater in this setting, requiring shorter follow-up intervals and more judicious use of PET imaging to distinguish fibrosis from tumor. Ultimately preventing progressive fibrosis is the best defense for preventing LC in patients with pulmonary fibrosis.
Obstructive sleep apnea and lung cancerSleep disordered breathing (SDB) has been shown to increase cancer incidence and mortality in several studies [47,48]. A large Spanish cohort of more than 4900 individuals found that the risk of cancer was more than double for those who spent 12% or more of their time asleep with an oxygen saturation below 90% (T90). The association was more pronounced in men younger than 65 [49]. Similar results were found after more than 20 years of follow up of the Wisconsin Sleep Cohort, with cancer mortality quintupled for individuals with severe obstructive sleep apnea (OSA). An 8-fold increase in cancer mortality was seen for those with the largest hypoxemia index or T90 [50]. Similarly, a large French study found that the incidence of cancer after 6 years of follow up was greater in patients with nocturnal hypoxemia. In that study the association was more pronounced in obese, older patients who did not comply with or receive adequate OSA therapy, and correlated with both lung and breast cancer [51].
Nocturnal hypoxemia is a common finding in patients with OSA and tobacco related diseases such as COPD and emphysema, and may be the key variable fueling oncogenesis, tumor progression and cell proliferation. It has also been described as a predictor of LC incidence and progression in experimental models [52,53]. Intermittent hypoxemia in both in vitro and in vivo studies appear to promote tumor growth, angiogenesis, and even resistance to therapy [54]. As with other chronic respiratory disease, several biological mechanisms have been proposed to justify this association, including altered immunity, inflammation, oxidative stress, and endothelial dysfunction [55]. Disruption of circadian rhythms may also play a role [55].
The association between nocturnal hypoxemia and cancer may be especially relevant for smoking related tumors such as LC [56]. In fact, propensity score matching of patients originating from two separate studies of SDB including patients with established LC and those at high risk for it enrolled in a LC screening program revealed a powerful link between nocturnal hypoxemia and LC [57]. Interestingly, in the LC screening cohort of patients who met National Lung Screening Trial screening criteria coupled with at least one additional known risk factor (COPD or emphysema) for LC, nocturnal hypoxemia predicted positive screening findings, meaning that individuals at risk for LC were more likely to harbor suspicious lung nodules if they suffered from significant nocturnal hypoxemia [58].
Subsequent work has revealed that moderate-to-severe OSA is linked to increased levels of biomarkers of immune evasion, lymphangiogenesis, and tumor aggressiveness including PSPC-1, MDK, PD-L1, PD-1, and TGF-β1 [59]. Furthermore, elevated plasma levels of Galectin-9 and TIM-3 in this setting are associated with a four-fold higher risk of mortality and larger tumor size in patients with LC [60]. Galectin-9 and TIM-3 play a key role in immune evasion and may be considered potential biomarkers of prognosis as well. They have been linked to inflammation and are inversely correlated with T-cell infiltration and proliferation. Because these biomarkers are also elevated in patients with OSA without cancer, it is likely that they provide a favorable milieu for tumors to appear. Finally, combined PD1 and TIM-3 inhibition have been shown to be synergistic in anti-cancer immunotherapy, so ongoing trials of anti-TIM-3 inhibition may offer promising breakthroughs in this setting for patients with OSA and LC [61,62]. Intriguingly, we have yet to investigate whether standard therapy for OSA (CPAP) and/or nocturnal oxygen therapy may have an impact on LC incidence in patients with moderate or severe nocturnal hypoxemia. Of note, although they are prospective and high-quality studies, they are observational, show no causal relationship, and many have inclusion biases (smoking, COPD, etc.). Therefore, future studies must contend these competing and co-existing causes of death, which are also independently associated with a greater LC risk.
Bronchiectasis and lung cancer riskThe association between BE and cancer is a newcomer to this field, with the first European report published by McDonnell et al. [63], showing that solid and hematological malignancies were the most frequent cause of death in BE patients. This risk was reported to be particularly high for lung and esophageal cancer with a prevalence of 5% and 3.5%, respectively.
Sin et al. [64] also investigated the causes of death in 18,134 BE patients in comparison with 90,313 non-BE patients. They observed that cancer was the first cause of death in BE patients causing 31.2% of all deaths, including 12.4% due to LC alone. BE patients had an increased risk of cancer with an adjusted HR of 3.36 (95% CI; 2.18–5.18).
A more recent systematic review by Castaldo et al. [65] identified 8 studies published between 2014 and 2022 including 202,681 BE patients. Overall, the authors reported a prevalence ranging from 0.93% to 8.0%, and an incidence rate of 3.96, concluding that BE represents an independent risk factor for LC. Other LC risk factors identified in this population, included age, male gender, tobacco use and COPD. An association between COPD, BE and LC was also described by Chung et al. (adjusted HR 1.14; 95% CI 1.04–1.26) [66] with contradictory results reported by Kim et al. [67]. However, it is important to highlight the important limitations in this systematic review [65]. Seven out of the 8 retrospective studies were performed in Asia (5 in Korea and 2 in Taiwan), with potential sources of bias, due to the patient heterogeneity and different standards of care for this condition in different countries. The only European study included in the review published by Sanchez-Carpintero et al. [68] did not find a significant difference in LC incidence or prevalence in BE patients compared to controls in a chest CT based LC screening program. An unpublished report by Gotera et al., did however, report an increased incidence of LC in patients with COPD and BE enrolled in a LC screening program in Spain.
BE patients’ characteristics, are extremely heterogeneous, with variable prevalence of smoking, tuberculosis, and genetic conditions that could favor the development of BE (i.e.: primary dyskinesia, immune-deficiencies and cystic fibrosis) and severely affect the reliability of data regarding the association between BE and LC.
There are some specific pathological conditions associated with BE that could increase the risk of LC, such as nontuberculous mycobacterial lung disease (NTMLD) [69]. Immune-deficiencies that cause a variable proportion of BE have also been associated with an increased cancer risk [70]. Apart from the typical infectious complications, primary immune-deficiencies (PID) are also characterized by an immune dysregulation that could lead to allergy, autoimmunity, lympho-proliferation and neoplasia. In fact, cancer is one of the leading causes of death in these patients, including lymphoma and gastric carcinoma, but also LC. Different factors have been considered to increase the risk of cancer in PID such as impaired immune response, DNA repair defects, deficient apoptosis, reduced immune surveillance [71] and protection from infections (e.g., human herpes virus 8 and Kaposi sarcoma) that are potentially carcinogenic [70].
In summary, substantial evidence has accrued in the last decade supporting an increased risk of LC in patients with BE. Although the evidence of the association between BE and LC is increasing, the quality and strength of the available data does not support the recommendation of including BE patients in LC screening programs.
ConclusionThe most prevalent chronic respiratory diseases (COPD, emphysema, asthma, ILD and OSA) not only commonly coincide but they are also considered independent risk factor for LC (Table 1). They shared common risk factors such as Inhalation of toxic tobacco smoke, fine particulate matter or occupational exposure in the context of a favorable genetic milieu, but also pathophysiology mechanisms such as hypoxemia, chronic inflammation, as well as altered immune surveillance that have also been proposed as potential partners in causality (Fig. 1). This, along with the significant improved survival in many of them, suggest that their inclusion in LC screening programs with low dose chest CT could be considered to improve their detection and survival.
FundingNone.
Conflicts of interestLuis M. Seijo is part of the Editorial board of Archivos de Bronconeumología and declares that they have remained outside the evaluation and decision-making process in relation to this article.
The rest of the authors declare no conflict of interest.