Cystic fibrosis (CF) is a rare genetic autosomal recessive disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene [1]. This muco-obstructive lung disease is accompanied by repeated cycles of inflammation with excessive neutrophil infiltrates and infections (e.g. Pseudomonas aeruginosa (Pa)), leading to remodelling of the respiratory epithelium [2]. Highly effective modulator therapies, e.g. elexacaftor/tezacaftor/ivacaftor (ETI), have considerably changed the life expectancy of people with CF (pwCF) in medicalised countries with an impressive improvement in CF clinical outcomes [3]. Data concerning a potential effect of ETI on airway epithelial cell alterations in pwCF are still scarce and mainly studied in vitro[4,5]. CC16 protein is the most abundant secreted protein in lung fluids associated with club cells. CC16 has anti-inflammatory, antioxidant, and immunomodulatory properties. In CF, a lower level of CC16 was observed in bronchoalveolar lavage fluid (BALF) from pwCF compared to people with chronic bronchitis [6] and in sputum from pwCF in stable and exacerbation phases compared to healthy controls [7–9]. Our hypothesis was that CC16 could be a potential indicator of the reversal of epithelial alterations and be an easily quantifiable protein in bodily fluids from pwCF. The aim of this study was firstly to assess CC16 expression in bronchial tissues and in human bronchial primary epithelial cultures (HBEC) to evaluate the alterations on CC16-positive cells in tissues remodelled by CF end-stage disease. Secondly, we compared CC16 concentration in sputum, serum, and urine from controls (CTRL) and pwCF without ETI (w/o ETI group) and with ETI (ETI group), to evaluate the effect of CFTR modulators on CC16 expression.
This work comprised a cohort study on residual human body material approved by the ethical committees of Cliniques Universitaires Saint-Luc (2007/19MARS/58) and KULeuven (S51577, S5217, S55877), and an interventional study, approved by the hospital Medical Ethics committee of Cliniques Universitaires Saint-Luc (Brussels, Belgium) (2015/09JAN/014), in which each participant signed an informed consent. Lung tissues used for immunohistochemistry and HBEC were obtained from surgical tissue from patients undergoing lobectomy for solitary nodule (remote from the tumour) or from unused lungs from non-smoker donors for the CTRL group, and from explants (at end-stage disease) for the CF group. For bodily fluids analyses, CF participants in stable condition were recruited during a routine visit at the CF Reference Centre of the Cliniques Universitaires Saint-Luc while the CTRL group consisted of non-smokers with no diagnosis of lung disease and a normal spirometry. Additional details are available as supplementary information (Table S1, primer sequences).
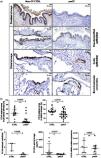
Lung tissues were obtained from 33 pwCF (32 tissue explants and one lobectomy), and 32 CTRL obtained during lung tumour surgery (n=24) and from unused lung donors (n=8). All CTRL have a Forced Expiratory Volume in 1s/Forced Vital Capacity (FEV1/FVC) ratio above 70% (characteristics in Tables S2–S4). The percentage of CC16-stained area was significantly lower in pwCF than in non-smoker CTRL in the proximal airways (median CTRL=42% and pwCF=4%, p=0.0005) and, in the distal airways (median CTRL=50% and pwCF=37%, p=0.0018) characterised by either pseudostratified epithelium (median CTRL=52%, and pwCF=25%, p=0.0016) or bronchoalveolar connection (median CTRL 49%, and pwCF=30%, p=0.0180) (Figs. 1a-b and S2a). No significant differences were observed for the cubic epithelia in the distal airways (Fig. S2b).
In primary cultures, the number of CC16-stained cells was lower in HBEC from pwCF (median CTRL=3cells/field and pwCF=0.05cells/field, p=0.0498, Fig. 1c). The release of CC16 was also lower in HBEC from pwCF than in CTRL (median CTRL=560pg mL−1 and pwCF=48pg mL−1, p=0.0047, Fig. 1c). No significant differences were observed for CC16 mRNA expression (Fig. 1c).
Lower expression of CC16 in lung explant tissues and in air–liquid interface (ALI) cultures of bronchial epithelial cells from people with cystic fibrosis (pwCF) at end-stage disease compared with tissues from non-CF controls (CTRL). (A) Representative images of CC16 immunostaining in the respiratory tract of CTRL and pwCF, (b) quantification of CC16-stained area in the proximal airways of 14 CTRL and 19 pwCF, and in the distal airways of 19 CTRL and 27 pwCF, (c) quantification in ALI-HBECs of CC16-positive cells per field (3 fields/culture, 3 CTRL and 4 pwCF), of CC16 protein release in the apical lavage (8 CTRL and 8 pwCF), and of CC16 mRNA expression normalised to the geometric mean of three housekeeping genes (7 CTRL and 10 pwCF). Mann–Whitney U test. Data are expressed as median±interquartile range.
Between 10th March 2022 and 9th May 2023, sputum, urine and serum were collected from 87 participants (flowchart in Fig. S1), including 57 clinically stable pwCF with or without ETI at enrolment, and 30 CTRL, matched for age and sex (characteristics listed in Tables S2–S4).
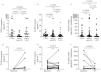
Sputum CC16 concentration was lower in pwCF w/o ETI compared with CTRL (median CTRL=0.9μg mL−1 and pwCF w/o ETI=0.2μg mL−1, p=0.0455). Sputum CC16 concentration was higher in pwCF with ETI compared with pwCF w/o ETI (median w/o ETI=0.2μg mL−1 and ETI=0.9μg mL−1, p=0.0055), reaching the level observed in CTRL (p>0.9999) (Fig. 2a).
Restoration of CC16 concentration in sputum from people with cystic fibrosis (pwCF) with elexacaftor/tezacaftor/ivacaftor (+ETI) compared with pwCF without ETI treatment (w/o ETI) and non-CF healthy controls (CTRL), increase in CC16 sputum level and decrease in CC16 urine level after ETI initiation. (a) CC16 concentration in sputum from 17 non-CF healthy controls (CTRL), 15 pwCF w/o ETI and 20 pwCF with ETI (Kruskal–Wallis test and Dunn's test), (b) CC16 concentration in serum from 29 CTRL, 29 pwCF w/o ETI and 46 pwCF with ETI (Kruskal–Wallis test and Dunn's test), (c) CC16 concentration in urine from 30 CTRL, 29 pwCF w/o ETI and 46 pwCF with ETI (Kruskal–Wallis test and Dunn's test), (d) CC16 expression in sputum from 7 pwCF before and after ETI initiation (Wilcoxon signed rank test), (e) CC16 expression in serum from 19 pwCF before and after ETI initiation (Wilcoxon signed rank test), and (f) CC16 expression in urine from 19 pwCF before and after ETI initiation (Wilcoxon signed rank test). Data are expressed as median±interquartile range.
No significant differences between groups were observed in serum (Fig. 2b) nor urine (Fig. 2c). No significant correlations were observed between CC16 levels in sputum, urine, and serum and clinical data nor neutrophil elastase (NE) concentration, except a weak correlation between serum CC16 and percent predicted FEV1 (ppFEV1) or maximal mid-expiratory flow in pwCF with ETI (r=0.337, p=0.022 and r=0.399, p=0.006 respectively) (Fig. S4). Despite the absence of correlation between CC16 level in sputum and NE activity, we observed a degradation of CC16 after 4h of incubation with NE (Fig. S5), linking high neutrophilic inflammation and CC16 decrease. Comparison of paired values before and after treatment initiation showed a significant increase of sputum CC16 levels (median at baseline=0μg mL−1, and after ETI initiation=0.9μg mL−1, p=0.0312, Fig. 2d) but not in serum CC16 (Fig. 2e). Urine CC16 concentration decreased after initiation of ETI (median at baseline=97.6μg mL−1 and after ETI initiation=20.0μg mL−1, p=0.0290, Fig. 2f).
Through a multimodal approach, we report a lower CC16 expression in lung tissues of CF end-stage disease compared with CTRL, persisting in post-epithelial dedifferentiation in vitro at protein level. Furthermore, a lower sputum CC16 concentration characterises pwCF without CFTR modulator treatment, while ETI initiation restores it.
CC16 quantification in airway tissues from pwCF shows that the number of CC16-stained cells in proximal and distal airways of pwCF is reduced. As previously showed with ciliated cells [10], the loss of CC16-stained cell is also maintained in vitro, suggesting an imprinted defect of CC16-stained cells in pwCF albeit with no mRNA alteration, suggesting post-transcriptional regulation. These decreases in CC16-stained cells highlight the major CF airway remodelling, as previously described at end-stage disease [10], combined with high neutrophilic inflammation further decreasing CC16 levels in sputum. This is of particular interest at a time when revolutionary CF treatments are questioning the established concept of irreversible airway remodelling. Indeed, some studies support the reversal of bronchiectasis after ETI initiation [11–13], but none concerning the reversal of airway epithelial alterations.
The lower level of CC16 in sputum from pwCF compared with CTRL confirms several studies conducted in induced sputum [9], spontaneous sputum [8] and bronchoalveolar lavage fluid (BALF) from pwCF [6]. In our study, no significant correlation between sputum CC16 and lung function was observed, corroborating a previous study [9]. However, a marginal correlation between CC16 levels in BALF and FEV1 of children with CF was reported [6]. Contrary to other studies, no significant correlation was observed between sputum CC16 levels and CRP [6] or NE activity and CC16 levels were not associated with Pa infection [7]. Although we showed a degradation of CC16 by NE, this absence of correlation and the decrease in HBEC (in which no NE is present) suggest that both neutrophilic inflammation and CFTR dysfunction, and the subsequent epithelial remodelling play a role in the decrease of CC16 in pwCF. Our study showed an association between serum CC16 with lung disease severity, as previously shown in children with CF [14], contrary to others [7].
Secondly, we investigated whether ETI could restore or at least increase CC16 levels in bodily fluids from pwCF. Our study shows an increase in sputum CC16 levels with ETI confirming a recent sputum proteome analysis [15]. ETI appears to promote lung CC16 secretion, enabling it to be restored to levels similar to those in CTRL. It will subsequently be interesting to assess the influence of ETI on the morphology of the respiratory epithelium in vitro. Recently, Hampton and colleagues demonstrated gene expression changes in HBEC from pwCF exposed to ETI for 48h, with a particular decrease in matrix metalloprotease genes playing a key role in lung damage [16]. This observation could be consistent with a change in physiology and remodelling of the respiratory epithelium.
For the first time, we demonstrate a decrease in urine CC16 of pwCF following the initiation of ETI. This observation could reflect improved reabsorption of low-molecular-weight proteins in the kidney from pwCF after ETI initiation and reinforce the potential role of CFTR in proximal tubule endocytosis [17].
This study has several limitations. CC16 quantification was performed on tissues from pwCF at end-stage disease prior to lung transplantation, not reflecting the global CF population, and an age difference was observed between the groups, although the age was not correlated with the CC16 staining results. In addition, serum samples were taken throughout different times of the day, yet serum CC16 levels vary according to the circadian cycle [18], potentially explaining the serum CC16 results. For sputum collection, we induced sputum in CTRL but not in pwCF. However, no significant differences in the composition of induced and spontaneous sputum were reported to date [19]. In our study, SCGB1A1 genetic polymorphisms were not evaluated and could explain the inter-individual variations observed [14]. Finally, sweat tests were not carried out in parallel with this work to assess a correlation between CFTR function and CC16 levels.
In conclusion, CC16 could represent an indicator of reversal of epithelial alterations, easily quantifiable in sputum from pwCF. Furthermore, our results support the important role of club cells in CF lung pathophysiology.
CRediT authorship contribution statementAngélique Mottais: investigation; writing – original draft, review & editing; methodology; supervision; data curation; validation; visualisation.
Bruno Detry: methodology; investigation; writing – review & editing.
Ziyu Alessia Qiu: methodology; data curation; investigation; writing – original draft, review & editing.
Amandine M. Collin: methodology; investigation; writing – review & editing.
Marylène Lecocq: methodology; investigation; writing – review & editing; supervision.
Caroline Bouzin: methodology; software; validation; writing – review & editing.
Clara Chamlou: investigation; data curation; writing – review & editing.
Chloé Bruart: investigation; data curation; writing – review & editing.
Charlotte de Fays: data curation; writing – review & editing.
Bart Vanaudenaerde: writing – review & editing.
Lieven Dupont: writing – review & editing.
Astrid Vermaut: writing – review & editing.
Marianne Schulte: data curation; writing – review & editing.
Mieke Boon: writing – review & editing.
Christophe Goubau: investigation; writing – review & editing.
Silvia Berardis: investigation; writing – review & editing.
Valérie Hox: writing – review & editing.
Antoine Froidure: writing – review & editing.
Charles Pilette: writing – review & editing.
Sophie Gohy: funding acquisition; methodology; supervision; data curation; validation; visualisation; writing – original draft, review & editing.
Declaration of generative AI and AI-assisted technologies in the writing processArtificial intelligence has not been used.
FundingThis work was supported by the Fund for Scientific Research – FNRS (grant 1.R016.18).
Conflicts of interestS. Gohy is recipient of the Clinical Researcher fellowship of the Fund for Scientific Research – FNRS (grant 1.R016.18). A. Mottais and S. Gohy received a Vertex Pharmaceuticals grant for a different research project. M. Boon received speaker fees from Vertex Pharmaceuticals for another project. All others authors declare no competing interests.
We would like to thank Pr Benoit Rondelet and Pr Valérie Lacroix (Departments of Thoracic Surgery of CHU Mont-Godinne and the Cliniques Universitaires St-Luc, Belgium, respectively) for their help in the tissue sample collection. We would also like to thank the nurses and physiotherapists at the Cystic Fibrosis Reference Centre of the Cliniques Universitaires Saint-Luc for their invaluable help in the samples collection. Finally, we would like to thank Céline Bugli of the Statistical Methodology and Computing Service at UClouvain for her help in the statistical analyses.