The importance of exercise in cystic fibrosis (CF) is well known since regular training is able to reduce lung function decline and to improve exercise tolerance and quality of life.1 Moreover, exercise can enhance muco-ciliary clearance by inhibiting epithelial sodium channel (ENaC) conductance, decreasing Na re-absorption and favouring airway fluid secretion.2 Therefore, current international guidelines recommend it as a physiotherapy adjunct to improve the efficacy of airway clearance techniques (ACTs).3
Beyond that, little is known about the effect of physical activity on inflammation and immune defence mechanisms in CF patients; only few studies are available, focusing mainly on pulmonary physiotherapy techniques.4 Differently, some evidences are available in other diseases: in chronic obstructive pulmonary disease (COPD) training programs have demonstrated to exert a positive effect on lymphocyte proliferative response.5 Studies on mice models showed that moderate exercise could induce a shift in immune response from a Th1 to a Th2 response.6 This change seemed to be associated with reduced viral or bacterial load, inflammation, infection-related morbidity and mortality.6,7
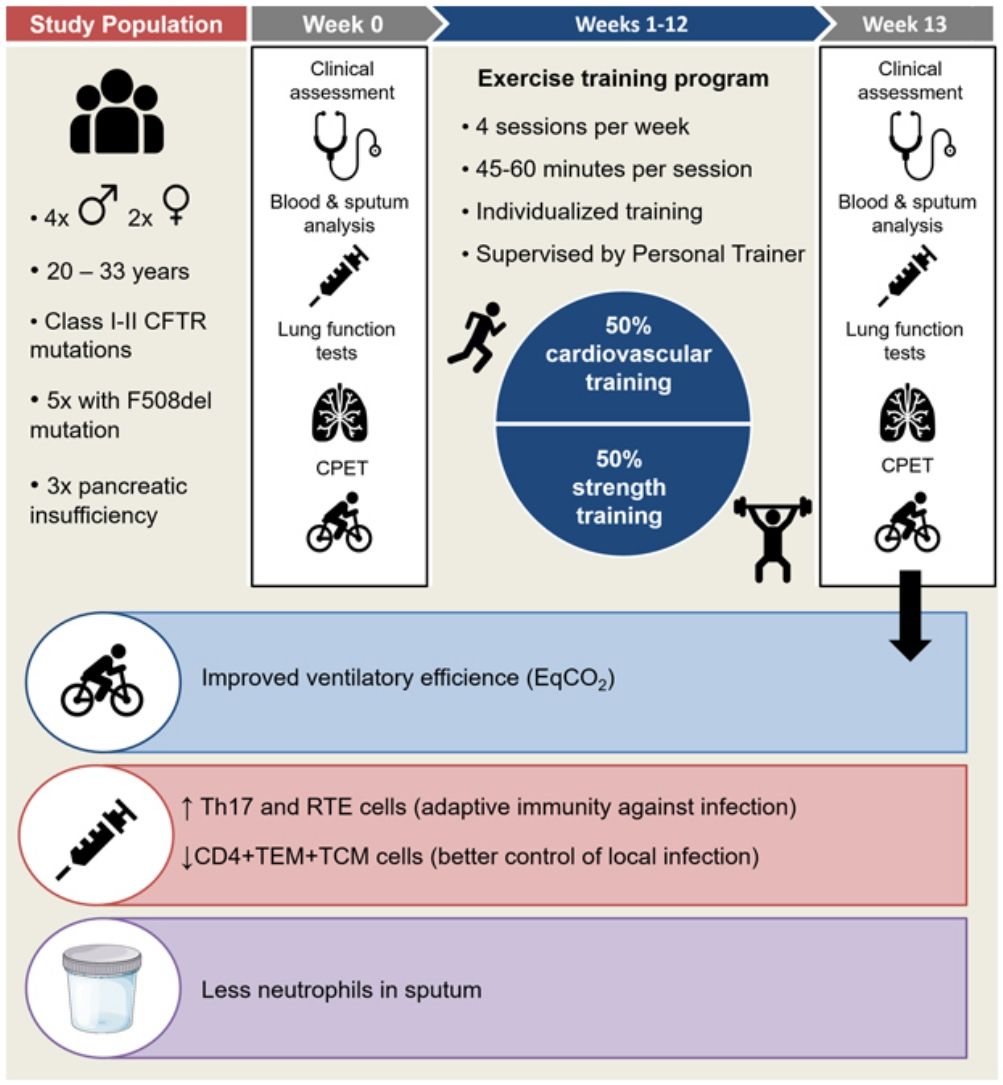
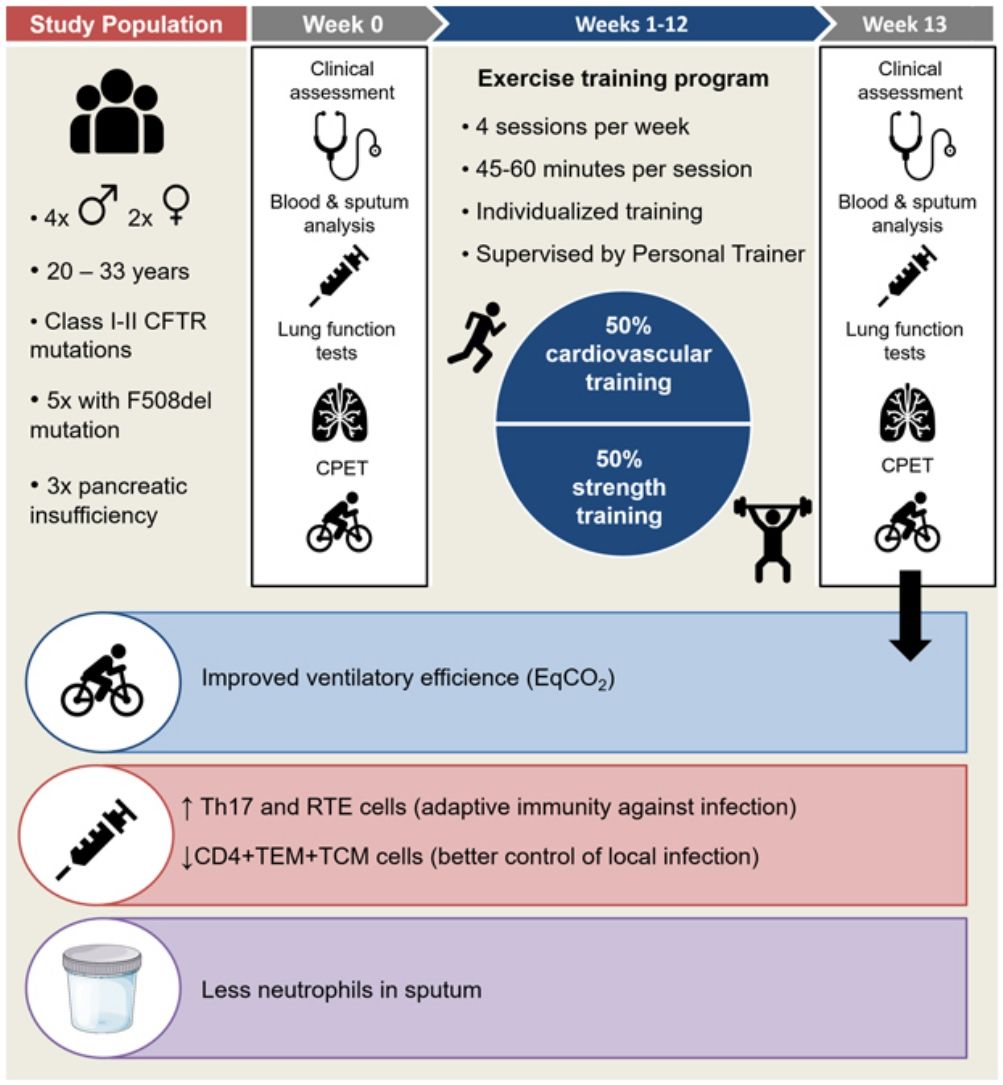
We conducted a prospective interventional single-centre pilot study in the Cystic Fibrosis Unit of our Hospital from February to July 2019, to investigate whether an exercise program can exert a modulator effect on immune system of adults with CF and chronic bronchial infection (CBI).
Inclusion criteria were: stable adult CF patients with CBI, body mass index (BMI)>19, forced expiratory volume in one second (FEV1) between 30% and 90% of predicted value, exclusion of immunological or autoimmune disorders. Written informed consent to participate in the study was obtained from participants. Ethics approval was granted by the Ethics Committee of our Hospital.
Exercise training program: A 12-week individualized training program in a dedicated gym under the supervision of a professional personal trainer was planned, with 4 sessions per week of 45–60min each (adapted to individual physical capability), focused on cardiovascular resistance and strength. A minimum of 3 sessions/week was required to patients. Individual starting capacity for the exercise training program was based on the evaluation of physical fitness during the first week of training and the exercise test at first visit. Patients were instructed how to perform the exercises at the start of the study and their progression was evaluated by the personal trainer once a week. The personal routine was mostly organized in order to train between 60% and 75% of maximal initial effort, based on baseline exercise test, and to have 50% of training dedicated to cardiovascular resistance and 50% to strength, but personalization of the training program was set by the trainer according to individual characteristics.
Within 7 days before the training, protocol included clinical and functional assessment, complete lung function tests (forced spirometry, diffusion capacity of the lungs for carbon monoxide –DLCO- and plethysmography), cardiopulmonary exercise test (CPET), blood and sputum collection. The same procedures were scheduled one week after the end of the training in order to avoid the effects of acute immune response.
For statistical analysis, data normality was checked using Kolmogorov–Smirnov and Pearson omnibus normality tests. For data comparison, Paired t test was used to compare parametric data, and the Wilcoxon-Mann–Whitney test was used to compare non-parametric data. Significance was set at P<0.05. GraphPad Prism 8.0 package (GraphPad Software, Inc, USA) was used.’
Six patients were recruited, two female and four males, aged 20-33 years (mean 26.17±5.23), all with class I or II CFTR mutations and 3 with pancreatic insufficiency (F508del was present in 5 out of 6 patients, being homozygosis in only 1 patient). Four patients were on chronic treatment with fluticasone (100 to 500mcg/daily) in combination with salmeterol, while 2 patients only used salbutamol on demand. Mean FEV1 was 57.2±23.6% of predicted, DLCO/VA was 97.7±17.7%, Total Lung Capacity (TLC) 104.2±10.5%.
CBI was due to methicillin-sensitive Staphylococcus aureus (MSSA) and Pseudomonas aeruginosa (PA) in 5/6 and 4/6 patients respectively. Three patients had both MSSA and PA chronic infection. The target for completion of the training was of 4 sessions per week during the 12 weeks of the study (100%). A specific questionnaire demonstrated a mean completion of the training of 74±10.25% (standard deviation). Only one patient poorly followed the program due to fact he doubled his workload in a car service achieving similar training effects; therefore he was maintained in the study for observational purpose. Analysis of advanced peripheral blood immunophenotype was performed by flow cytometry.8 At the same time, spontaneous (5 patients) or induced (1 patient) sputum was collected and processed for differential leukocyte count by microscopy.
One patient with chronic MSSA and PA infection had a mild exacerbation at week 10 of training, treated with amoxicillin/clavulanate for 8 days. During that period her training programme was suspended. This complication did not impact on her FEV1 or on her capacity of exercise; unfortunately, it was not possible to analyse blood or sputum during exacerbation.
At the end of the training period, lung function tests did not show significant changes.
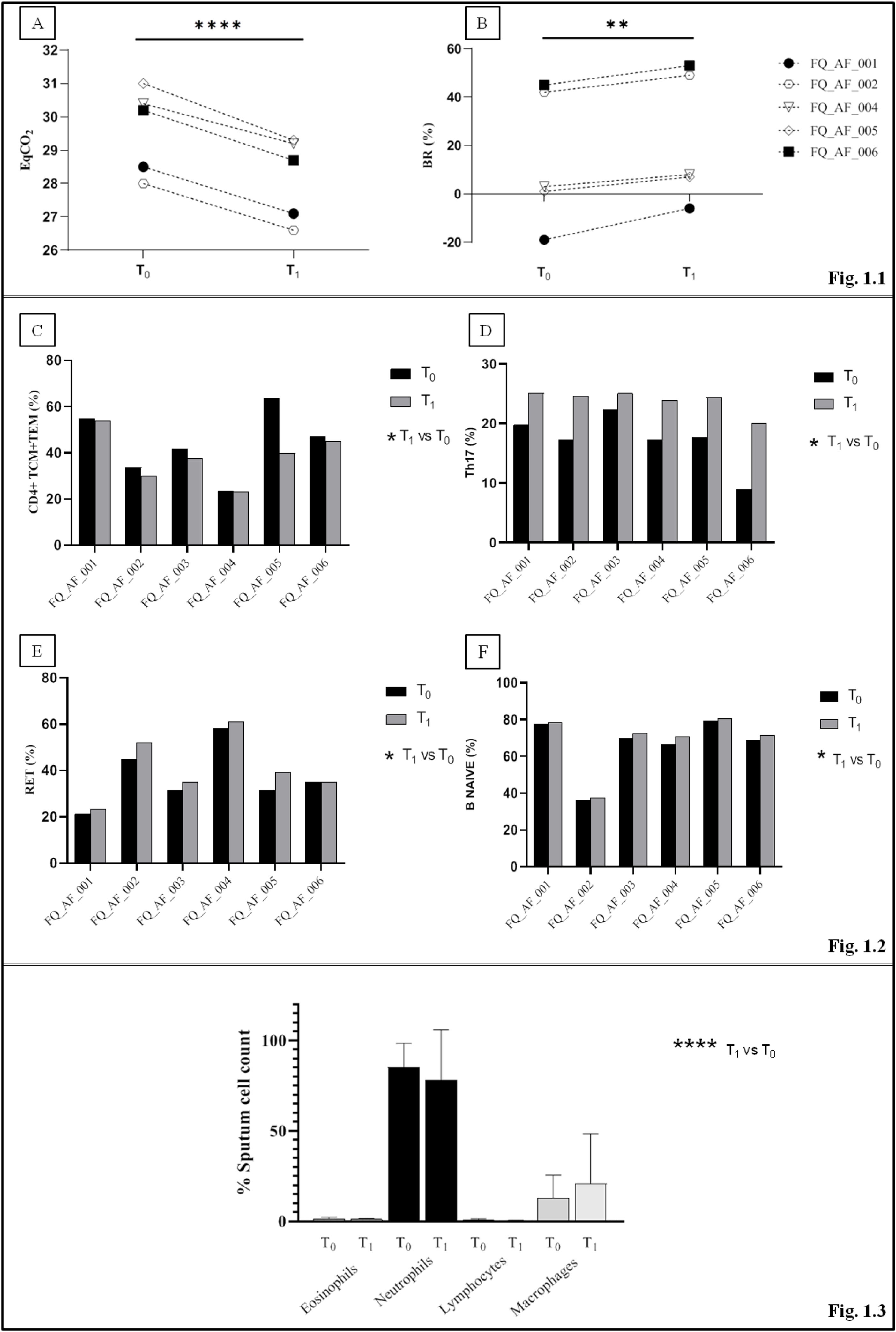
In comparison with baseline, post-training CPET showed a significant decrease in ventilatory equivalent for carbon dioxide (EqCO2) from 30.1% to 28.7% (p<0.0001), while breathing reserve (BR) increased significantly in all subjects (10.2% to 24.5%, p=0.002)(Fig. 1.1).
The figure represents only the variables showing significant changes between T0 (baseline) and T1 (end of the study). (1.1) Parameters of cardiopulmonary test at T0 and T1 measure points. Five patients are represented in the figure since one patient (FQ-AF-003) did not achieve maximal effort exercise at T1. (A) The ventilatory equivalent for carbon dioxide (EqCO2) significantly decreased while (B) the Ventilatory reserve (BR) significantly increased. (1.2) Percentages of T and B cells in peripheral blood samples before and after the training period for each subject. Blood lymphocytes subpopulations are represented: (C) A significant decrease in the percentage of CD4+ effector memory (TEM) and central memory (TCM) T cells at T1 was observed. Th17 subpopulation (D), recent thymic emigrant cells population (RET) (E) and B CD4+naïve subpopulation (F) showed a mild increase at the end of the training period (T1). (1.3) Results of sputum cell count at T0 and T1. Neutrophils and macrophages significantly predominated over eosinophils and lymphocytes at both T0 and T1. P values for T1 -vs- T0 comparisons are represented as follows: * p<0.03; **p=0.002; **** p<0.0001.
In peripheral blood samples, no significant changes were found in the main lymphocyte subpopulations. However, a significant decrease in the percentage of CD4+ effector memory (TEM) and central memory (TCM) T cells was observed after training (44.0–38.16%, p<0.03). The analysis of T-helper cell functional subsets showed a significant percentage increase in Th17 subset cells (17.2–23.9%; p<0.03), while Th1 and Th2 tended to decrease (37–30.6%; p=0.18 and 33.6–20.3, p=0.19 respectively) and Th1-17 showed a non-significant increase (14.5–25.2%; p=0.10). Recent thymic emigrant cells (RTEs) significantly increased in all subjects (36.9–40.9%; p<0.03) such as the B naive subpopulation (IgD+CD27−) from 66.4% to 68.6% (p<0.03) (Fig. 1.2).
Sputum cell differential count showed significant predominance of neutrophils and macrophages over other cell types in both phases of the study (p<0.0001). Compared to baseline, no significant differences were detected after the training (p=0.99), but mild reduction in neutrophils was observed (−7%, Fig. 1.3).
In conclusion, as expected, our pilot study confirms that training was able to improve efficacy of ventilation by increasing BR and decreasing EqCO2.
Beyond that, we also found interesting changes in blood lymphocytes subpopulation after training. A significant increase of the Th17 cells was observed in peripheral blood; these cells play a role in adaptive immunity against pathogens, especially PA,9 maintaining mucosal barriers and contributing to pathogen clearance.10 In particular, there is clear evidence about the role of Th17 cells in modulating both systemic and airways inflammation in CF.11,12 On the other hand, the observed increase of RTEs may indicate a moderate expansion of the T-naive cell repertoire (CD45RA+ CCR7+) (that in fact increased in 4 patients), which represents a positive effect on the immune response,13 while the reduction of TEM and TCM may favour a better control of the local infection.
Lastly, the mild reduction in neutrophils observed in sputum suggests that exercise might be capable of reducing neutrophilic inflammation by improving airways clearance and ventilatory efficacy.
This pilot study has several limitations, including: limited sample size, lack of a control group, small number of measures, absence of absolute cellular count.
Despite that, our preliminary trial may suggest that also the immune system is positively influenced by exercise training. This new research hypothesis deserves in our opinion further investigation particularly by increasing sample size, in order to finally improve CF outcomes in the future.
Conflict of interestsEva Polverino has received consultancy and speaker's fees from: Bayer, Grifols, Insmed, Chiesi, Menarini, Zambon, Pfizer, Teva, Shire and Polyphor. The remaining authors have no conflicts of interest.
The authors sincerely thank Prof. R Pujol Borrell for his advice and review of the manuscript.