The interviewer-administered chronic respiratory questionnaire (CRQ-IA) is widely used and has demonstrated excellent properties for measuring health-related quality of life (HRQL) in patients with chronic obstructive pulmonary disease (COPD). However, the self-administered version (CRQ-SAS) in Spanish has not been validated.
The aim of this trial was to evaluate the validity and the sensitivity of the Spanish version of the CRQ-SAS in patients with COPD.
Material and methodsWe randomized 40 patients with COPD (33 treated with pulmonary rehabilitation and 7 with liquid oxygen therapy) to one of the two methods of administration of CRQ (SAS vs IA) both before and 8 weeks after the treatment. In addition, patients completed the SF-36 questionnaire, pulmonary function tests, and 6-min walk test.
ResultsThe CRQ-SAS demonstrated good longitudinal construct validity on all domains with a range of correlations, for the change scores, between 0.46 (P=.05) and 0.71 (P=.01). Regarding sensitivity to change, we observed a minimal clinically significant change in most domains (fatigue 0.71 [P=.02], emotional factor 0.62 [P=.04], and control of the disease 0.83 [P=.06]).
ConclusionsThe Spanish version of CRQ-SAS is valid for evaluating HRQL in COPD patients. The correlations of the CRQ-SAS with other tools provide construct validity and show good sensitivity to change.
El cuestionario de la enfermedad respiratoria crónica con entrevistador (CRQ-IA) ha demostrado excelentes propiedades de medida de la calidad de vida relacionada con la salud (CVRS) en pacientes con enfermedad pulmonar obstructiva crónica (EPOC). La validación en español de la versión estandarizada autoadministrada (CRQ-SAS) no se ha realizado. El objetivo de este estudio fue evaluar la validez y la sensibilidad a los cambios, de la versión española del CRQ-SAS en pacientes con EPOC.
Pacientes y métodosAleatorizamos a 40 pacientes con EPOC (33 en tratamiento con rehabilitación respiratoria y 7 con oxígeno líquido) a uno de los dos métodos de administración del CRQ (SAS vs. IA) antes y 8 semanas después de la intervención. Los pacientes completaban el cuestionario SF-36, pruebas de función pulmonar y prueba de los 6min de marcha.
ResultadosEl CRQ-SAS ha demostrado una buena validez de constructo longitudinal en todas las áreas con un rango de correlaciones, para las puntuaciones del cambio, entre 0,46 (p=0,05) y 0,71 (p=0,01). En cuanto a la sensibilidad a los cambios, se observa un cambio mínimo clínicamente significativo en la mayoría de áreas (fatiga 0,71 [p=0,02], factor emocional 0,62 [p=0,04], control de la enfermedad 0,83 [p=0,06]).
ConclusionesLa versión española del CRQ-SAS resulta ser válida para evaluar la CVRS de los pacientes con EPOC. Las correlaciones con otros instrumentos aportan validez de constructo y se demuestra una buena sensibilidad a los cambios.
Health-related quality of life (HRQL) is an important tool used to evaluate the impact of therapeutic interventions on health in most chronic diseases.1,2
In general, the questionnaires that measure HRQL have been developed in English and have been adapted to different languages. In order to use these questionnaires in other countries with languages other than English, not only is a translation/retro-translation, but also studies demonstrating their validity, reliability and sensitivity to changes are necessary. The most widely used questionnaires in patients with COPD are the Saint George's respiratory questionnaire (SGRQ),3 the chronic respiratory disease questionnaire (CRQ)4 and the SF-36 health questionnaire (SF-36).5
The original interviewer-administered version of the chronic respiratory disease questionnaire (CRQ-IA) was developed for its application in patients with COPD and was designed to evaluate the impact of certain interventions including, pharmacological treatments, respiratory rehabilitation, oxygen therapy, etc.6,7 This instrument has demonstrated its usefulness in a great variety of studies, with adequate discriminative properties and great sensitivity to changes. The Spanish version of the CRQ-IA was translated and validated some years ago4,8 and since then it has been widely used not only in Spain but also in other Spanish-speaking countries. The Spanish version of the CRQ-IA also shows good discriminative and evaluative properties for COPD patients under different treatments such as pulmonary rehabilitation,9 and home oxygen therapy.10
However, the interviewer version (CRQ-IA) has some points that could limit its use. The individualized dyspnea domain limits the possibility of comparing among patient groups and programs,6,11 in addition to requiring a trained interviewer. In recent years, the authors of the original CRQ have developed a self-administered standardized version of the CRQ (CRQ-SAS) with the aim of simplifying its use and also allowing for comparisons between populations. This self-administered version has been demonstrated to be valid in various languages, such as English,12 French13 and German.14 In all these settings, the CRQ-SAS has demonstrated excellent validity and sensitivity to changes compared with the CRQ-IA or with other instruments, in addition to significantly reducing the time required for its administration.15
The objective of this study was to analyze the validity and the sensitivity to change of the Spanish version of the CRQ-SAS in the evaluation of the HRQL of COPD patients.
Materials and MethodsStudy DesignFrom September 2004 to September 2006, we developed a randomized study to demonstrate the validity of the self-administered version of the CRQ-SAS in a group of patients diagnosed with COPD before and after treatment with lung rehabilitation or liquid oxygen.
The patients were randomized in centralized blocks by one of the authors of the questionnaire in Canada (HS). They were given either the self-administered version of the CRQ-SAS or the CRQ-IA with the questions on dyspnea domain of the standardized version. The questionnaires were administered during the first week after selection (baseline) and then after finalizing the respiratory rehabilitation program or 2 months after initiating treatment with liquid oxygen (follow-up).
The protocol was approved by the ethics committee of the hospital and all the patients signed the informed consent.
PatientsThe patients were selected consecutively from among the patients usually seen in our center who had been diagnosed with COPD in accordance with the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD)16 who had been proposed for either a respiratory rehabilitation program or for being treated with liquid oxygen.
We included patients whose mother tongue was Spanish who were under the age of 75 with a grade of dyspnea measured by the Medical Research Council (MRC)>2.17 The patients chosen had been clinically stable without episodes of exacerbation in the last month or without changes in the previous 4 months.
Excluded from the study were those patients with reading or writing difficulties, with osteomuscular, cardiac or respiratory (other than COPD) diseases that limited their exercise capacity.
Rehabilitation ProgramThe rehabilitation program included groups of 8–10 subjects each and had a duration of 8 weeks. The patients attended rehab for 3h, 3 days a week. The program consisted of:
- 1.
Education about the disease and the treatment, with 4 sessions directed at patients and their family members.
- 2.
Respiratory physiotherapy including directed ventilation and bronchial drainage (if indicated) techniques, as well as relaxation techniques.
- 3.
Muscle training, including exercises for the upper and lower extremities and the respiratory muscles. The muscles of the upper limbs were exercised with weights (initially with 500g for each limb) in 30min sessions. The weight was increased by 1kg each week until the patients reached the maximum tolerable weight. The muscles of the lower extremities were exercised with a cycle ergometer, also in 30-min sessions, with an initial load representing 60% of the maximum load reached during an incremental effort test. The load was increased depending on the tolerance of the patient. For training the respiratory muscles, 15min sessions were carried out with a threshold valve device (Threshold, Respironics, Cedar Grove, New Jersey, USA), with an initial inspiratory pressure corresponding to 30% of the maximal inspiratory pressure, with increments depending on the patient's tolerance.
The patients who complied with the criteria for home oxygen therapy with liquid oxygen18 performed a baseline 6-min walk test (6MWT), breathing room air to demonstrate the need for oxygen. Later, successive 6MWTs were done to adjust the oxygen flow necessary to reach SpO2≥90%. Patients had a break of at least 30min between tests. The degree of dyspnea was evaluated by means of the Borg scale,19 both at the beginning and end of the test.
The Spanish Version of the Chronic Respiratory Disease Questionnaire (CRQ-IA)The validation of the original version with an interviewer of this questionnaire was done in collaboration by a group of Spanish pulmonologists with the original author (GG) and was demonstrated to be valid for evaluating the HRQL in COPD patients in our setting.4,8
The CRQ includes 20 questions, distributed into 4 domains: dyspnea (5 questions), fatigue (4 questions), emotional factor (7 questions) and disease control (4 questions). The dyspnea area of the CRQ-IA is individualized, allowing the patient to choose between the 5 activities that cause him/her the most dyspnea. In follow-up testing of the CRQ, the patient evaluated his/her level of dyspnea related with said activities. The responses for each area are quantified in a 7-point Likert scale. The sum of points for each domain is divided by the number of questions, obtaining a value between 1 and 7 for each. The evaluation of the resulting HRQL ranges from 1 (maximum affectation of the HRQL) up to 7 (no affectation). A change of 0.5 points or more per area is considered the minimal clinically significant change.20
The Self-Administered Version of the Chronic Respiratory Disease Questionnaire (CRQ-SAS)The standardized dyspnea domain includes 5 questions that evaluate 5 activities that produce respiratory difficulties in most patients with COPD:
- 1.
Feeling emotions, such as anger or disgust.
- 2.
Personal hygiene (bathing, showering, eating or dressing).
- 3.
Walking.
- 4.
Performing routine daily activities (housework, shopping, errands, grocery shopping).
- 5.
Participating in social activities (meeting with family, friends, neighbors or groups).
The other 3 domains (fatigue, emotional factor and disease control) have the same content as in the CRQ-IA, but they are self-administered. The quantification is done in the same way as the CRQ-IA.
The transcultural adaptation of the CRQ-SAS questionnaire was only done for the dyspnea domain, as in the rest of the domains the content had not been modified from the original questionnaire. For this process, the same method was followed as for the original CRQ-IA questionnaire.4,8
CRQ Validation InstrumentsSF-36 Health QuestionnaireThis is a generic instrument for evaluating HRQL that includes 36 questions that measure eight areas: physical function, limitation due to health problems general vitality, pain, general perception of health, social function, limitation due to emotional problems and general mental health. The SF-36 score ranges from 0 to 100, with higher scores indicating better function and good state of health. SF-36 has demonstrated to be valid and sensitive to the changes in patients with chronic respiratory diseases, both in English21 as well as in Spanish.5
6-Min Walk TestWe used the 6MWT to evaluate the functional capacity for exercise in accordance with the ATS guidelines,22 including the measurement of oxygen saturation at the beginning and at the end of the test, with the calculation of the means. At the baseline determination, the test was repeated three times, either on consecutive days or after a rest period of at least an hour in order to take the best out of the three distances. The degree of dyspnea during effort was also evaluated by means of the Borg scale, at the beginning and the end of the test. An increase of at least 35m in the distance walked was considered the clinically significant minimal change.23
Statistical AnalysisThe variables used in the study were expressed as the mean and interquartile range as per description.
In order to calculate the mean difference between the baseline scores, the follow-up scores and the change between them, comparing the two different questionnaires (CRQ-SAS and CRQ-IA), the non-parametric Mann–Whitney test for independent samples was used due to the fact that the variables did not follow normal distribution. These are expressed as mean difference, as well as 95% confidence interval. This test was done to rule out the differences obtained in the scores having been due to chance.
For the construct and criteria validity, Spearman's correlation coefficient was calculated for the baseline scores (cross-sectional baseline), for the follow-up scores (cross-sectional follow-up) and for the change (longitudinal), for the different areas of the CRQ (SAS and IA), the SF-36 and the 6MWT.
To compare the sensitivity to changes of the CRQ-SAS and the CRQ-IA, the Wilcoxon test for paired samples was used. The results are expressed as means and 95% confidence interval. A change of 0.5 points or more, per area, was considered the minimal clinically significant change.20
All the analyses were done using the SPSS statistical program, version 15.0 (SPSS Inc., Chicago, IL, USA).
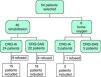
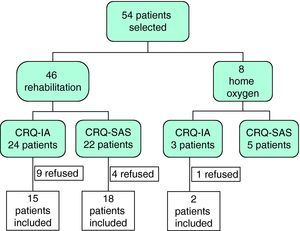
ResultsInitially, 54 patients were evaluated to participate in our study, 14 of whom rejected treatment: one patient refused home oxygen therapy and 13 abandoned the rehabilitation program (10 before initiating and 3 before finishing the program). Of the 40 remaining patients, 33 completed the rehabilitation program and 7 had therapy with liquid oxygen. All of them were randomly assigned to either the CRQ-IA (n=17) or the CRQ-SAS (n=23) groups (Fig. 1).
Table 1 shows the demographic and clinical characteristics of both groups. There are no statistically significant differences between both groups. Out of the patients with COPD, 15% were in GOLD stage II, 42.5% in stage III and 42.5% in stage IV.
Demographic Information of the Patients Who Completed the Self-Administered Version (SAS) and Interviewer-Administered Version (IA) of the CRQ.
| Characteristics of the Patients | CRQ-SAS | CRQ-IA |
| Patients, n | 23 | 17 |
| Oxygen therapy, n | 5 | 2 |
| Female sex, % | 2 (8.7) | 4 (23.5) |
| Age, years | 65±11 | 70±10 |
| FEV1% pred. | 29±26 | 43±29 |
| BMI | 26.7±4.9 | 27.5±3.8 |
| 6MWT | 285±167 | 300±89 |
6MWT: 6-min walk test; BMI: body mass index; CRQ: chronic respiratory questionnaire (CRQ); FEV1: forced expiratory volume in one second.
Values expressed as mean and interquartile range.
The patients of the rehabilitation group showed a clinical improvement and a statistically significant improvement in the distance walked during the 6MWT: 45.3m (P=.01) in the CRQ-SAS group and 49.7m (P=.00) in the CRQ-IA group.
In Table 2 we can observe the comparison between the scores of both questionnaires (CRQ-IA and CRQ-SAS) at baseline situation, follow-up and change, expressed as mean difference and 95% confidence interval. We found no statistically significant differences between the two questionnaires administered for the measurement of the HRQL in baseline situation, follow-up and change.
Baseline, Follow-up and Change Scores of the Self-Administered Version (SAS) and Interviewer-Administered Version (IA) of the CRQ.
| Area | Visit | CRQ-SAS×(95% CI) | Pa | CRQ-IA×(95% CI) | Pa | Mean Difference (95% CI) | Pb |
| Individualized dyspnea | n=17 | ||||||
| Baseline | 3.14 (2.73; 3.55) | ||||||
| Follow-up | 3.83 (3.25; 4.42) | ||||||
| Change | 0.69 (0.26; 1.13) | .004c | |||||
| Standardized dyspnea | n=23 | n=17 | |||||
| Baseline | 5.00 (4.40; 5.59) | 4.94 (4.18; 5.70) | 0.06 (−0.87; 0.99) | .79 | |||
| Follow-up | 5.26 (4.78; 5.74) | 4.93 (4.05; 5.81) | 0.33 (−0.54; 1.21) | .45 | |||
| Change | 0.26 (−0.21; 0.73) | .32 | −0.01 (−0.83; 0.80) | .97 | 0.27 (−0.56; 1.11) | .51 | |
| Fatigue | n=23 | n=17 | |||||
| Baseline | 3.93 (3.42; 4.45) | 4.00 (3.38; 4.62) | −0.07 (−0.84; 0.70) | .86 | |||
| Follow-up | 4.65 (4.20; 5.09) | 4.01 (3.30; 4.73) | 0.63 (−0.14; 1.41) | .12 | |||
| Change | 0.71 (0.19; 1.24) | .02c | 0.01 (−0.32; 0.35) | .93 | 0.70 (0.10; 1.31) | .02* | |
| Emotional factor | n=23 | n=17 | |||||
| Baseline | 4.58 (3.95; 5.21) | 4.39 (3.65; 5.14) | 0.19 (−0.75; 1.13) | .68 | |||
| Follow-up | 5.20 (4.73; 5.68) | 4.64 (3.90; 5.38) | 0.56 (−0.25; 1.38) | .17 | |||
| Change | 0.62 (0.03; 1.20) | .04c | 0.24 (−0.13; 0.62) | .14 | 0.37 (−0.35; 1.10) | .30 | |
| Disease control | n=23 | n=17 | |||||
| Baseline | 4.67 (3.97; 5.36) | 4.84 (4.13; 5.55) | −0.17 (−1.14; 0.80) | .72 | |||
| Follow-up | 5.50 (5.03; 5.97) | 4.95 (4.17; 5.74) | 0.54 (−0.30; 1.39) | .39 | |||
| Change | 0.83 (0.09; 1.57) | .06 | 0.12 (−0.21; 0.44) | .46 | 0.71 (−0.08; 1.51) | .08 | |
CRQ: chronic respiratory questionnaire (CRQ).
Tables 3 and 4 show the cross-sectional construct and criteria validity of the CRQ-IA and the CRQ-SAS at baseline situation and during follow-up. In general, we have observed moderate correlations of the CRQ with the other instruments used for the validation.
Cross-Sectional Criteria and Construct Validity of the Self-Administered Version (SAS) and Interviewer-Administered Version (IA) of the CRQ: Correlations for the Baseline Scores.
| Instruments | CRQ-SAS | CRQ-IA | |||||||
| Standardized Dyspnea | Fatigue | Emotional Factor | Disease Control | Individualized Dyspnea | Standardized Dyspnea | Standardized Dyspnea | Emotional Factor | Disease Control | |
| Individualized dyspnea | – | – | – | – | – | – | – | – | – |
| Standardized dyspnea fatigue | – | – | – | – | 0.55* | – | – | – | – |
| Emotional factor | 0.59** | – | – | – | 0.69** | 0.35 | – | – | – |
| Disease control | 0.42* | 0.52* | – | – | 0.36 | 0.44 | 0.71** | – | – |
| 0.56** | 0.39 | 0.62** | – | 0.37 | −0.22 | 0.59* | 0.49* | – | |
| SF-36, physical | 0.64** | 0.77** | 0.33 | 0.16 | 0.43 | 0.56* | 0.22 | 0.33 | 0.07 |
| SF-36, mental | 0.26 | 0.47* | 0.64** | 0.16 | 0.15 | 0.31 | 0.56* | 0.87** | 0.34 |
| 6min walking test | 0.32 | 0.30 | 0.23 | 0.11 | -0.03 | 0.32 | 0.40 | 0.53* | 0.31 |
CRQ: chronic respiratory questionnaire.
Spearman's correlation coefficient.
Cross-Sectional Criteria and Construct Validity of the Self-Administered Version (SAS) and the Interviewer-Administered Version (IA) of the CRQ: Correlation for the Follow-up Scores.
| Instruments | CRQ-SAS | CRQ-IA | |||||||
| Standardized Dyspnea | Fatigue | Emotional Factor | Disease Control | Individualized Dyspnea | Standardized Dyspnea | Fatigue | Emotional Factor | Disease Control | |
| Individualized dyspnea | – | – | – | – | – | – | – | – | – |
| Standardized dyspnea | – | – | – | – | 0.72** | – | – | – | – |
| Fatigue | 0.53* | – | – | – | 0.53* | 0.45 | – | – | – |
| Emotional factor | 0.06 | 0.53* | – | – | 0.43 | 0.28 | 0.91** | – | – |
| Disease control | 0.29 | 0.57** | 0.78** | – | −0.01 | 0.15 | 0.53* | 0.61** | – |
| SF-36, physical | 0.48* | 0.56* | 0.53* | 0.46* | 0.72** | 0.49 | 0.44 | 0.26 | −0.17 |
| SF-36, mental | 0.14 | 0.33 | 0.26 | 0.36 | 0.18 | 0.31 | 0.60* | 0.67** | 0.46 |
| 6 minute walk test | 0.46* | 0.17 | 0.01 | 0.15 | 0.04 | −0.02 | 0.47 | 0.47 | 0.47 |
CRQ: chronic respiratory disease questionnaire.
Spearman's correlation coefficient.
For the study of the longitudinal construct validity of the scores for change, we analyzed the correlations between the scores of change in the CRQ (CRQ-SAS and CRQ-IA) with the scores of change of the SF-36 and the 6MWT (Table 5).
Longitudinal Criteria and Construct Validity of the Self-Administered Version (SAS) and the Interviewer-Administered Version (IA) of the CRQ: Correlation for the Scores of Change (Follow-up/Baseline).
| Instruments | CRQ-SAS | CRQ-IA | |||||||
| Standardized Dyspnea | Fatigue | Emotional Factor | Disease Control | Individualized Disease | Standardized Dyspnea | Fatigue | Emotional Factor | Disease Control | |
| Individualized dyspnea | – | – | – | – | – | – | – | – | – |
| Standardized dyspnea | – | – | – | – | −0.18 | – | – | – | – |
| Fatigue | 0.54** | – | – | – | 0.28 | −0.39 | – | – | – |
| Emotional factor | 0.51* | 0.66** | – | – | −0.07 | 0.06 | 0.43 | – | – |
| Disease control | 0.50* | 0.46* | 0.69** | – | 0.26 | −0.14 | 0.25 | −0.17 | – |
| SF-36, physical | 0.24 | 0.30 | 0.32 | 0.12 | 0.39 | 0.05 | 0.02 | −0.47 | −0.03 |
| SF-36 mental | 0.45 | 0.52* | 0.71** | 0.61** | −0.43 | 0.45 | −0.34 | 0.83** | −0.39 |
| 6-min walk test | −0.26 | 0.03 | 0.22 | −0.02 | −0.01 | −0.26 | −0.28 | −0.33 | 0.07 |
CRQ: chronic respiratory disease questionnaire.
Spearman's correlation coefficient.
In the analysis of the criteria validity, the majority of the correlation coefficients for the scores of change were higher for the CRQ-SAS compared with the CRQ-IA. We found correlations from moderate to strong and statistically significant.
To evaluate the sensitivity to change of the CRQ, we compared the change produced between the baseline situation and follow-up for each questionnaire. The CRQ-SAS showed a statistically significant change in the areas of fatigue (3.93 vs 4.65, P=.02) and emotional factor (4.58 vs. 5.20, P=.04) and a minimal clinically significant change in the areas of fatigue (0.71), emotional factor (0.62) and disease control (0.83). However, the CRQ-IA demonstrated only a statistically significant change in the individualized area of dyspnea (3.14 vs 3.83, P=.004) that was also clinically significant (0.69) (Table 2).
DiscussionThe results of this validation study indicate that the CRQ-SAS presents good discriminative as well as evaluative properties for the measurement of the HRQL in patients with COPD. Contrary to previous studies15 and to our expectations, the CRQ-IA did not score well for its evaluative properties (sensitivity to changes and longitudinal construct validity) and we believe that this is the result of the lack of therapeutic response in the group of patients randomized to the CRQ-IA in this small study.
The randomized design of the study supports the homogeneity of the groups, thus if the patients administered the CRQ-SAS improved after rehabilitation, we found no explanation why those in the CRQ-IA group did not do so.
One important limitation of the study is the number of patients included in the final analysis as the results are based on 40 patients and on a mixed population (rehabilitation patients and those in treatment with oxygen therapy). It is probable that with a larger sample we would have observed an important and statistically significant improvement in all the areas in both questionnaires.
Another limitation of the study is that it did not compare the individualized with the standardized domain of dyspnea in the group of patients who did the CRQ-SAS. The individualized dyspnea domain showed a good sensitivity to the changes in the CRQ-IA group, but we cannot compare it with the standardized area of dyspnea of the CRQ-SAS.
Within the validation process, we have not considered it necessary to repeat the study of reliability nor viability as the content of the questionnaire is the same as the previously validated original.4,8 However, it is possible that it may be of interest to carry out this study with the aim of determining the behavior of this new version with regard to this psychometric property.
We also did not study the degree of agreement between the CRQ-IA and CRQ-SAS as this was demonstrated in the initial analysis done by the authors of both questionnaires.15 Perhaps it would be interesting to perform this study in the current Spanish version.
ValidityWe developed the CRQ-SAS in order to facilitate its administration and comparison between different situations and patient groups. In previous studies,14,15 the CRQ-SAS shows adequate construct and criteria validity, with an expected but small reduction in the sensitivity to changes compared with the CRQ-IA. Likewise, in the study by Holger et al.15 it was demonstrated that the self-administered version of the dyspnea domain of the CRQ-SAS improves the criteria validity, maintains the construct validity but reduces the sensitivity to changes compared with CRQ-IA. The German version of the CRQ-SAS, like this present study, only analyzed the construct validity. The authors demonstrated a better longitudinal construct validity for the CRQ-SAS, represented by greater correlations between the scores for change of the CRQ and those of other instruments of validation.14 In our study, we observed good correlations for the scores of change with all the areas of the CRQ-SAS and only with the physical component of the SF-36, but not with the 6MWT.
Our study demonstrates higher correlations between the scores of change of the CRQ-SAS with the changes in the other validation instruments compared with those of the CRQ-IA. Once again, the CRQ-SAS presents a better longitudinal construct validity than the CRQ-IA. These results indicate that the standardization of the dyspnea domain does not compromise said validity.
Sensitivity to ChangesIt was observed that the individualized questions of the dyspnea domain showed a greater sensitivity to changes in the CRQ-IA than the standardized questions of the CRQ-SAS. These results are similar to our previous studies.12 One possible explanation for this fact would be that with CRQ-IA, the patients choose the activities that cause them dyspnea. Therefore, the patients may be more alert to the changes in these activities after treatment, more than to the changes produced in the standard activities of the CRQ-SAS, which probably are not are not familiar to them.
The main limitation of the use of the individualized dyspnea questions is that they make comparisons among populations difficult. However, they are closer to the daily activity of the patients.
The sensitivity to changes in the other domains was superior for the CRQ-SAS compared with the CRQ-IA. One possible explanation would be that it was due to the sample size or simply to chance.
In summary, the Spanish version of the CRQ-SAS shows a moderate validity and a good sensitivity to changes, particularly for the areas of fatigue and emotional factor. However, although the sensitivity to changes for CRQ-SAS is clearly acceptable, in the individualized area of dyspnea of the CRQ-IA the sensitivity is higher.
Given our results, our opinion is that researchers should choose the CRQ version depending on the sample size with which they are working. For studies with extensive series, it would be preferable to choose the CRQ-SAS due to the fact that it is self-administered because the CRQ-IA requires a trained interviewer, despite both tools demonstrating equally excellent properties for measurement over time. Perhaps the optimal situation would be to use the CRQ-SAS but, for the individualized dyspnea domain, use the CRQ-IA given its greater sensitivity to changes, keeping in mind that comparisons could not be made between populations or treatments in the area of dyspnea, although they could in other domains.
In conclusion, the Spanish version of the CRQ-SAS is valid for evaluating the HRQL of COPD patients. The correlations with other instruments provide construct and criteria validity, as well as good sensitivity to changes.
Conflict of InterestsThe authors declare having no conflicts of interest.
The authors would like to thank Francesca Sperati for her collaboration in the statistical analysis of this present study.
Please cite this article as: Vigil L, et al. Validez y sensibilidad al cambio de la versión española autoadministrada del cuestionario de la enfermedad respiratoria crónica (CRQ-SAS). Arch Bronconeumol. 2011;47:343–9.
Supplementary data associated with this article can be found in the on-line version.