Previous studies have shown that physical activity (PA) in COPD is associated with a better quality of life and less morbidity and mortality. Our aim was to study the daily PA in the lives of stable COPD patients, outside the setting of a pulmonary rehabilitation program.
Materials and methodsObservational, descriptive and transversal multi-center study in patients with stable COPD controlled in an outpatient clinic by pneumologists. In order to determine the Physical Activity Index (PAI), the Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ) was used to differentiate the following groups according to the energy expenditure: inactive (less than 1000kcal/week), moderately active (between 1000 and 3000kcal/week) and very active (more than 3000kcal/week). We analyzed the relationship between PAI and disease severity, health level and socioeconomic variables of the patients.
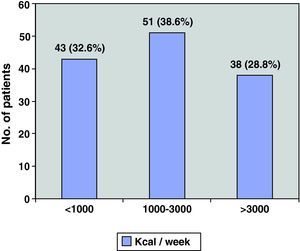
ResultsA total of 132 patients (121 men) were included in the study. Mean age was 66; mean FEV1 was 45%. Regarding PA, 32.6% had energy expenditures of less than 1000kcal/week, 38.6% between 1000 and 3000 and 28.8% more than 3000. The most inactive COPD patients had more bronchial obstruction, more severe disease, more dyspnea and walked fewer meters in the 6MWT.
ConclusionsStable COPD patients perform low levels of PA. Lower PA is associated with poorer health and with more severe disease.
Estudios previos han resaltado que la actividad física (AF) en la EPOC se asocia a mejor calidad de vida y menor morbimortalidad. Nuestro objetivo ha sido conocer los hábitos de AF en la vida diaria de pacientes EPOC estables fuera de un programa de rehabilitación respiratoria.
Materiales y métodosEstudio observacional descriptivo transversal multicéntrico en pacientes EPOC estables controlados ambulatoriamente por neumólogos. Para conocer el índice de AF (IAF) se utilizó el Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ), diferenciando según el gasto energético, los siguientes grupos: inactivos (menos de 1.000kcal/semana), moderadamente activos (entre 1.000 y 3.000kcal/semana) y muy activos (más de 3.000kcal/semana). Se analizó la relación entre el IAF y variables socioeconómicas, de severidad de la enfermedad y de nivel de salud de los pacientes.
ResultadosSe incluyó a 132 pacientes (121 varones). Edad media: 66.3 años, FEV1 medio 45%. Un 32,6% de ellos realizaba una AF menor de 1.000kcal/semana, un 38,6% entre 1.000 y 3.000 y el 28,8% más de 3.000. Los pacientes EPOC más inactivos, tenían mayor obstrucción bronquial, una enfermedad más severa, referían más disnea y caminaban menos metros en el 6MWT.
ConclusionesLos pacientes EPOC estables realizan un bajo nivel de AF. Una menor AF se asocia con un peor estado de salud y con una mayor gravedad de la enfermedad.
The importance of physical activity (PA) for health has been recognized and its beneficial effects have been demonstrated in primary as well as in secondary prevention of several chronic diseases.1,2
In COPD, more regular PA is associated with better quality of life3,4 and lower morbidity and mortality,5,6 and it is a predictive indicator for mortality in this type of patients.7 In addition, several studies dealing with respiratory rehabilitation (RR) programs have observed that patients who are more physically active show slower loss of lung function and fewer exacerbations of the disease.8,9
Despite this evidence, COPD patients show a clear tendency towards sedentary habits, which can be attributed to the bronchial obstruction as well as systemic repercussions10 and comorbidities that frequently accompany this pathology.11 The high prevalence of COPD12 together with the limited accessibility to RR programs in our setting means that very few patients can benefit from them. On the other hand, regular physical activity, theoretically, is within reach of the immense majority of patients.
In recent years, the importance of PA in the evolution of COPD has been emphasized13,14 and information on the transcendence of PA in this disease is growing,15 but our knowledge about the PA habits of COPD patients in their daily lives is still very limited.
The objective of this study is to learn about the daily physical activity habits of stable COPD patients outside of a respiratory rehabilitation program, also analyzing the variables associated with performing more or less physical activity.
Materials and MethodsCharacteristics of the StudyOurs is a multi-center, cross-sectional descriptive observational study of patients diagnosed with COPD, according to SEPAR criteria,16 in stable clinical situation and controlled in an outpatient setting by pulmonologists. We determined the physical activity index (PAI), and its relationship with several variables: socioeconomic, disease severity and level of patient health.
Characteristics of the PatientsThe patients were recruited consecutively from the outpatient pulmonology consultations of 6 hospitals in the Community of Valencia. They were classified by level of severity according to the SEPAR staging system16 and the BODE index.17 All the patients included in the study gave their written informed consent.
The inclusion criteria were the following: age between 40 and 80, post-bronchodilator FEV1 <80%, FEV1/FVC ratio <70 and clinical stability (defined as having elapsed at least 6weeks since the last exacerbation with no associated events).
We excluded those patients with one or more of the following circumstances: 1) participation in an RR program (neither at the moment of inclusion nor during the year prior); 2) analphabetism; 3) presence of active neoplasm or chronic invalidating disease that was an impediment for PA; 4) ischemic cardiopathy in unstable phase; and 5) plans to change residence in the following months.
MethodologyAll the variables were collected by means of a data form that included: 1) demographic data (age, sex), anthropometric data and body mass index (BMI) calculation; 2) degree of tobacco habit, calculating the number of pack-years; 3) degree of dyspnea according to the Medical Research Council (MRC) scale; 4) socioeconomic data (family status, occupation, economic and educational level); 5) associated comorbidities, calculating the Charlson index; 6) treatment; 7) questionnaires about the state of general, respiratory and mental health; and 8) questionnaire about the level of PA. Likewise, all patients underwent spirometry with a bronchodilator test and exercise capacity test (6-min walk test; 6MWT).
The data for general state of health were obtained by means of the SF12 questionnaire, those pertaining to respiratory health with the St. George's questionnaire and those for mental state with the depression-anxiety questionnaire (HD).
In order to quantify PA, the Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ) was used, which evaluates the physical activity performed by men and women in their spare time. This questionnaire compiles the PA performed during both the week and the year prior to data register. It is a tool that has been validated for the Spanish population18,19 and is able to estimate the energy output (EO) for each type of physical exercise, determining the PAI expressed in MET (metabolic equivalent of task). MET is the energy output for the metabolic rate at rest, which in adults equals 3.5ml of oxygen per kilogram of weight per minute or 1kcal per kilo of weight and per hour or 1kcal/min. In order to estimate the energy output of each PA, a code system was used, known as the compendium of physical activities,20 which codifies each type of activity by its function, specific characteristics and intensity. This compendium includes activities related with the personal hygiene, leisure time, recreation, occupation and rest. This code assigns to each PA a unit of intensity based on the rate of energy output and it is expressed in MET. The intensity of energy assigned to each PA has been obtained from standardized experimental situations. It is based on the proportion between resting metabolic rate (RMR) and basal metabolic rate (BMR), expressed by the RMR/BMR ratio. It is assumed that 1 unit of intensity is equal to 1kcal/min (1 MET). The intensity of each PA is classified as multiples of a MET: an activity of 2 METs requires 2 times the metabolic energy output at rest. The physical activity ratio was calculated in kcal/day for each patient, according to the following formula: PAI=I×N×T, where I represents the code for the intensity for each PA in kcal/min, N the number of times that that PA is performed in a certain period of time, and T the time in minutes spent in each session.
Given that the recommended daily energy output for health should be at least 150–400kcal/day, and that it is considered that an increase in PA of 1000kcal/week is beneficial for COPD patients regarding a reduction in risk of mortality of 20%,21 in our study we have differentiated three levels of activity: 1) inactive patients (those who performed a weekly energy output of less than 1000kcal/week); 2) moderately active (those who performed between 1000 and 3000kcal/week); and 3) very active (patients with an energy output of higher than 3000kcal/week).
Statistical AnalysisWe completed a descriptive analysis of all the variables. In the study of the different levels of activity, we used the chi-square test for evaluating the association between the qualitative variables and Student's t-test for evaluating the association between the quantitative variables.
In the correlation study, the main dependent variable was PAI. The independent variables were socioeconomic, those related to the severity of the disease and those for the state of health of the patients. A linear model based on the one-way variance analysis (ANOVA one way) was used for the categorical variables, representing the means of the PA variable within each level of the variable, as well as the degree of statistical significance associated with this contrast. When the independent variable was continuous, a linear model was calculated based on the univariate linear regression with a representation of both the correlation coefficient and the P value associated with the contrast. A P value <.05 was considered statistically significant, and SPSS Statistics 17.0 software was used.
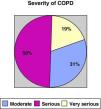
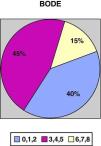
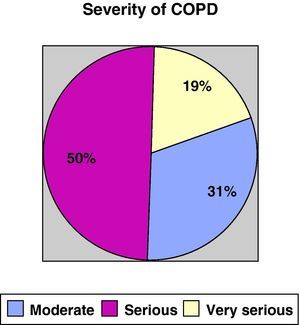
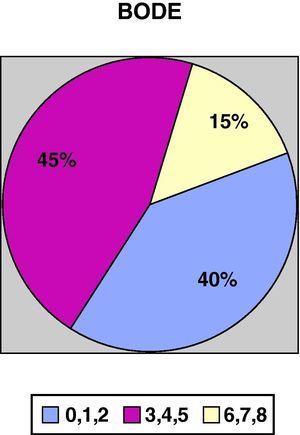
ResultsWe recruited a total of 132 patients, 91.7% men (121/132) and 8.3% women (11/132), with a mean age of 66.3±10 and a BMI of 28.13±5.5. With regards to tobacco habit, 82.4% were ex-smokers and 12.2% active smokers with a mean consumption of 52.17±26 pack-years. Mean FEV1 (% reference value) was 45±13.6; mean distance walked during the 6-min walk test was 364.5±99m. Figs. 1 and 2 show the distribution of the patients according to the severity of their COPD, depending on FEV1 and the BODE index, respectively.
COPD severity according to the SEPAR staging system.16
COPD severity according to the BODE index.17
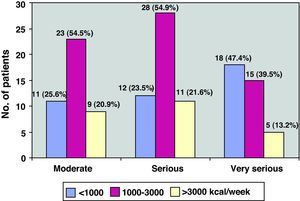
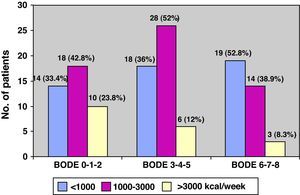
As for physical activity, 32.6% of the patients (43/132) performed PA of less than 1000kcal/week, 38.6% (51/132) between 1000 and 3000, and the remaining 28.8% (38/132) more than 3000kcal/week (Fig. 3). Fig. 4 shows the PA levels according to COPD severity levels classified in accordance with the SEPAR staging system, while Fig. 5 demonstrates the level of PA according to the BODE index.
The socioeconomic and tobacco use characteristics are reflected in Table 1. The low level of education stands out, as do a mid-to-low income level, a high proportion of retired persons and 12% of active smokers, although we did not find significant differences for any of these variables for level of PA.
Clinical characteristics and degree of tobacco habit according to level of PA.
| All | <1000kcal/week | 1000–3000kcal/week | >3000kcal/week | P | |
| No. of patients | 132 | 43 | 51 | 38 | |
| Age, years | 66±10 | 68.07±9.8 | 66.22±10.92 | 64.39±8.9 | .26 |
| Sex | 91.7% males | 93% males | 90.2% males | 92.1% males | .86 |
| BMI | 28.1±5.5 | 29.49±6 | 26.81±5.1 | 28.36±5.28 | .06 |
| Dyspnea, MRC | 1.79±0.85 | 2.02±0.89 | 1.84±0.73 | 1.47±0.89 | .01* |
| Smoking habit | |||||
| Non-smoker | 7 (5.3%) | 1 (2.3%) | 4 (7.8%) | 2 (5.3%) | .12 |
| Smoker | 16 (12.1%) | 8 (18.6%) | 5 (9.8%) | 3 (7.9%) | |
| Ex-smoker | 108 (81.8%) | 33 (76.7%) | 42 (82.4%) | 33 (86.8%) | |
| Pack-years | 52.1±26.6 | 54.48±25.3 | 49.83±31 | 52.83±21.1 | .71 |
| HOT | 45 (34.3%) | 16 (37.2%) | 20 (39.2%) | 9 (24.3%) | .31 |
| NIV | 15 (11.4%) | 4 (9.5%) | 5 (9.8%) | 6 (15.8%) | .56 |
| Family status | |||||
| Lives alone | 17 (12.9%) | 4 (9.3%) | 9 (17.6%) | 4 (10.5%) | .11 |
| Lives with spouse/partner | 99 (75%) | 29 (67.4%) | 38 (74.5%) | 32 (84.2%) | |
| Lives with family members | 15 (11.4%) | 9 (20.9%) | 4 (7.8%) | 2 (5.3%) | |
| Income level | |||||
| Low | 36 (27.3%) | 14 (32.6%) | 15 (29.4%) | 7 (18.4%) | .07 |
| Medium | 91 (68.9%) | 28 (65.1%) | 34 (66.7%) | 29 (76.3) | |
| High | 4 (3%) | 2 (3.9%) | 2 (5.3%) | ||
| Education | |||||
| No schooling | 33 (25%) | 11 (25.6%) | 12 (23.5%) | 10 (26.3%) | .33 |
| Primary school | 64 (48.5%) | 23 (53.5%) | 25 (49%) | 16 (42.1%) | |
| Secondary school | 23 (17.4%) | 7 (16.3%) | 9 (17.6%) | 7 (18.4%) | |
| Higher education | 9 (6.8%) | 1 (2.3%) | 4 (7.8%) | 4 (10.5%) | |
| Occupation | |||||
| Retired | 104 (78.8%) | 35 (81.4%) | 40 (78.4%) | 29 (76.3%) | .43 |
| Employed: sedentary work | 17 (12.9%) | 4 (9.3%) | 9 (17.6%) | 4 (10.5%) | |
| Employed: moderately active work | 10 (7.6%) | 4 (9.3%) | 2 (3.9%) | 4 (10.5%) | |
| Employed: strenuous work | 1 (0.8%) | 1 (2.6%) | |||
Some type of comorbidity was present in 65.2% of patients (85/132). There was one comorbidity in 29.5%, two in 31.1% and more than two in 4.5%. The most frequent were: HTA in 42 (31.8%), diabetes in 32 (24.2%) and cardiopathy in 22 patients (16.7%).
Regarding pharmacological treatment, 87% of the patients received a combination of inhaled corticosteroids and long-acting beta-adrenoceptor agonists (LABA), 81% tiotropium and 72.5% the triple-association of these medications. 34.1% of the patients (45/132) received home oxygen therapy and 11.4% (15/132) non-invasive ventilation. We found significant differences neither between the 3 levels of PA and the presence or absence of comorbidities nor between the three levels of PA and the type of treatment that the patients were receiving.
Table 2 shows the data of the health questionnaires and the COPD severity classification, including the BODE index, specifically according to the level of PA. As can be seen, there is a statistically significant relationship between the better scores for general health (SF12, P=.01) and respiratory questionnaires (St. George's, P=.03), fundamentally for the subscales for activity and impact as well as the increased level of PA. Fig. 5 illustrates the decline in physical activity as the BODE index increases. We did not detect a relationship with the state of mental health (HD questionnaire) and the level of PA. By the same measure, we found a statistically significant inverse relationship between disease severity, determined by FEV1 as well as by the BODE index and the level of PA. Independently, each of the variables that make up this index, with the exception of BMI, also showed a clearly significant inverse relationship with the level of PA. In short, the most inactive COPD patients had greater bronchial obstruction (P=.02), more severe disease (P=.03), reported more dyspnea (P=.01) and walked less meters in the 6MWT (P=.02) (Table 2).
Level of PA and its relationship with the degree of health and COPD severity.
| All | <1000kcal/week | 1000–3000kcal/week | >3000kcal/week | P | |
| No. of patients | 132 | 43 | 51 | 38 | |
| State of health | |||||
| SF12 | 37±4.8 | 35.28±4.5 | 37.73±5 | 37.97±4.48 | .01* |
| St. George | |||||
| Total score | 41.44±18.6 | 47.99±19.1 | 41.1±16.9 | 37.97±4.48 | .03* |
| Symptoms | 41.99±21.1 | 42.26±17 | 41.40±21.47 | 42.40±24.6 | .97 |
| Activity | 56.6±24 | 65.60±23 | 58.32±21.46 | 45.99±24.3 | .00* |
| Impact | 32.5±19.2 | 39.14±20.6 | 31.34±17.83 | 35.67±18.5 | .05* |
| SHD | |||||
| Anxiety | 4.8±4 | 14±5.5 | 4.48±3.7 | 4.7±4 | .48 |
| Depression | 4.32±3.8 | 16±5.1 | 4.1±3.45 | 3.7±3.6 | .27 |
| FEV1% | 45%±13.6 | 43±12.5 | 42.92±13.2 | 49.97±14.5 | .02* |
| BODE score | 3.42±1.88 | 3.86±2.12 | 3.52±1.7 | 2.78±1.7 | .03* |
| Degree of COPD | |||||
| Moderate | 41 (31.1%) | 11 (25.6%) | 12 (23.5%) | 18 (47.4%) | .06 |
| Severe | 66 (50%) | 23 (53.5%) | 28 (54.9%) | 15 (39.5%) | |
| Very severe | 25 (18.9%) | 9 (20.9%) | 11 (21.6%) | 5 (13.2%) | |
| 6MWT | 364.5±9.2 | 338.9±102 | 360.59±87 | 399.73±103 | .02* |
The correlation analysis showed a significant association between PAI over the previous year and the variables that reflected less COPD severity, as well as with living alone, being retired and being of a lower income level (Table 3).
Relationship between the physical activity in both time periods (weekly and annual) with COPD socioeconomic and severity variables.
| PA week | PA year | |||
| Mean/r | P | Mean/r | P | |
| Age | 0.126 | .151 | 0.217 | .013 |
| BMI | 0.034 | .696 | 0.032 | .717 |
| Dyspnea, MRC | ||||
| Grade 0: without dyspnea or at maximum effort | 3800.17 | .250 | 186 755.67 | .001* |
| Grade 1: dyspnea walking up gradual inclines | 2685.93 | 129 780.56 | ||
| Grade 2: dyspnea while walking quickly on the level | 2228.54 | 105 258.88 | ||
| Dyspnea walking on the level, less than 100m | 2035.38 | 660 940.17 | ||
| Tobacco habit | ||||
| Non-smoker | 2824.79 | .485 | 128 147.86 | .814 |
| Ex-smoker | 2497.14 | 110 505.51 | ||
| Smoker | 1746.22 | 103 174.13 | ||
| Pack-years | 0.020 | .829 | 0.023 | .803 |
| FEV1% | 0.152 | .082 | 0.244 | .005* |
| Degree of COPD | ||||
| Moderate FEV1 >50<80 | 2900.45 | .268 | 136 449.88 | .046* |
| Severe FEV1 >30<50 | 2273.44 | 102 059.80 | ||
| Very severe FEV1 <30 | 1952.22 | 878 830.8 | ||
| BODE index | ||||
| 0–1–2 | 2694.00 | .433 | 135 448.61 | .005* |
| 3–4–5 | 2076.60 | 989 660.25 | ||
| 6–7–8 | 2348.47 | 669 720.68 | ||
| 6MWT | 0.257 | .004* | 0.342 | .000* |
| Family status | ||||
| Lives alone | 2285.88 | .107 | 122 690.21 | .039* |
| Lives with spouse/partner | 2633.75 | 116 420.61 | ||
| Lives with family members | 1186.87 | 584 630.87 | ||
| Income level | ||||
| Low | 2557.26 | .433 | 870 620.58 | .024* |
| Medium | 2305.50 | 116 032.69 | ||
| High | 3885.50 | 198 679.00 | ||
| Education | ||||
| No schooling | 2594.00 | .682 | 112 880.06 | .200 |
| Primary school | 2235.15 | 101 541.53 | ||
| Secondary school | 2365.98 | 107 880.65 | ||
| Higher studies | 3254.22 | 165 824.00 | ||
| Occupation | ||||
| Retired | 2404.84 | .682 | 106 338.83 | .032 |
| Employed: sedentary work | 2068.03 | 876 270.35 | ||
| Employed: moderately active work | 2761.90 | 171 364.00 | ||
| Employed: strenuous work | 4892.00 | 234 816.00 | ||
Our study demonstrates that stable COPD patients who are seen in the outpatient pulmonology consultations of our hospitals, and who had not been included in any type of respiratory rehabilitation program, present a low level of physical activity. More specifically, one-third are inactive, almost 40% perform moderate exercise and only 30% have an energy output in line with the recommendations for obtaining a beneficial effect in their health and a reduction in the risk for morbidity and mortality. The most inactive COPD patients have greater bronchial obstruction, report more dyspnea, walk less meters in the 6MWT and have a poorer quality of life.
Our interest in this study was to analyze PA in a sample that was as representative as possible of stable COPD patients with different levels of severity. Thus, they were recruited from the outpatient consultations of various hospitals in the Community of Valencia, who span several degrees of health-care complexity, as reported in previous studies by our workgroup.22
The profile of these patients reveals a predominance of men, with high BMI, older mean age and either severe (50%) or very severe (19%) COPD, frequently accompanied by comorbidity (65%), usually cardiovascular. From a socioeconomic standpoint, there is a high proportion of retirees with limited income and limited education, most of whom live with either their spouse or family members. We cannot avoid the fact that 12% of these patients still continue to smoke. This description agrees with data previously published in Spain by Esteban et al.23 as well as by the extensive EIME study,24 which is considered to be a true reflection of the type of COPD patients who are seen in the pulmonology consultations. The inclusion of few women in the study, which of course was unintentional, does not enable us to draw conclusions about PA in this sex.
Although there is evidence of the beneficial effect of PA in all the stages of COPD, as reflected in the recommendations of the main clinical practice guidelines,16,25 there is little information about the PA performed by this type of patients. Therefore, and given that the severity of our patients oscillated from moderate to very severe COPD (FEV1 range from 58.6% to 31.4%), we distinguished 3 levels of PA: a group of very active patients (energy output greater than 3000kcal/week), another with an energy output in accordance with the recommendations1,2 on the minimum volume of PA that is beneficial for health at around 150–400kcal/day, and finally a group of clearly inactive patients.
Our study has confirmed that the limited PA of these patients is related with the perception of a low level of general and respiratory health as well as with several COPD severity indicators, both for the analysis of individualized variables (dyspnea, FEV1, 6MWT) and for the BODE index and its multidimensional evaluation of the disease.
In recent years, there has been a growing interest in the literature for learning about the characteristics of the PA in COPD patients. Different authors26–28 have underscored the marked daily inactivity in this type of patients, compared with that of control subjects with similar biological conditions. Likewise, they have observed that PA diminishes after GOLD severity stage II, although the clinical characteristics and the level of COPD severity did not always fully represent the degree of limitation of the daily activity of the patients.27,28
In Spain, there are two previous studies14,29 also analyzing the relationship between the level of PA and the state of health and severity of COPD patients. Our data agree with those of these authors regarding the quality-of-life variables support the fact that, in COPD patients who do not participate in a respiratory rehabilitation program, a higher level of PA is related with a better score in the general and respiratory health questionnaires. Likewise, both García-Aymerich et al.14 and Esteban et al.29 find a statistically significant relationship between the level of PA and various quality-of-life variables. Particularly, in the study by Esteban et al.,29 the patients were followed for 5years and the authors demonstrated that the health-related quality of life (HRQL) improved in those patients who increased their level of PA; meanwhile, the HRQL worsened in those who either maintained a low level of PA or reduced their level of PA over time.
Quite probably, the reasons for which PA improves the HRQL of COPD patients are based on the same multifactorial mechanisms which are attributed to the benefit of PA in the general population (improved cardiac function, improved peripheral muscle function and increased maximal oxygen consumption/intake, among others). They would also explain why, according to our results, the degree of dyspnea and the SF12 questionnaire (P=.01) are variables that show a greater degree of statistical significance related with level of PA.
Regarding the relationship between PA and COPD severity, the results of the different studies are not as homogeneous. Specifically, García-Aymerich et al.,14 unlike our results, found no relationship between the level of PA and disease severity, although their COPD patients were more severe than ours (means FEV1 35%), came from tertiary hospitals and had had several re-hospitalizations. In fact, their patients were recruited after one of these exacerbation episodes. The study by Esteban et al.29 included patients with different stages of severity (50% GOLD stage II, 44% GOLD stage III and 6% GOLD stage IV), somewhat less severe than our subjects. The authors state, in agreement with our results that a low level of PA is accompanied by greater dyspnea and greater COPD severity. Given that the quantification of the level of PA in the different studies was performed by the individualized administration of a specific questionnaire and that both García-Aymerich and we used the same one, it may be argued that perhaps the discrepancy in the results is due to the difference in the level of severity of the patients.
Certainly, there are currently more precise methods for determining the level of PA than simply obtaining data using questionnaires. Specifically, the use of pedometers and accelerometers can reflect the daily reality of patient mobility with greater accuracy. Particularly, accelerometers have been recommended for use in COPD patients as they can detect ranges of physical activity from light to intense,30 but the price of these devices is not insignificant, even more so in the context of a multi-center study in which not all participating hospitals can absorb such an investment. On the contrary, the MLTPAQ questionnaire validated for the Spanish population by Elosua et al.18,19 compiles an extensive list of possible activities carried out by the patient, for a short period (1week) as well as a long period (1year), excluding the seasonal bias and obtaining a global evaluation of the energy output performed by our patients. In fact, despite the differences in the method of determining the level of PA, our data also agree with those provided in previous studies.26–28 These authors used devices based on the incorporation of accelerometers and energy output sensors that measured the PA of the patients during the period of examination, and like us, they also detected a decrease in the PA in COPD patients from the early stages of the disease. This limitation was related with dyspnea, greater severity of the disease and with the decrease in walking capacity as evaluated by the 6MWT. Meanwhile GOLD stages III and IV were the best predictors for very inactive patients.27
Thus, despite the possible limitation of the study that can be involved in using the MLTPAQ questionnaire compared with other more precise methods for determining PA, we think that the results that we present in this study are valid and highly consistent. Furthermore, we feel that there are two aspects that should be mentioned. First of all, as ours is a multi-center study carried out by a recently-created COPD workgroup,22 it covers a wide number of hospitals with different degrees of health-care complexity, meaning that our results reflect the reality of the COPD patients usually seen in pulmonology consultations. Second, the fact that data were obtained for the PA of the patients for a short period (1week) as well as a long period (1year) enables us to obviate inaccuracies due to forgetfulness in the first case, while spanning the different seasons of the year in the second case, eliminating seasonal PA bias due to meteorological reasons.
Home oxygen therapy (HOT) and/or non-invasive ventilation (NIV) can have repercussions on patient PA levels. In our study, we found no relationship between the use of HOT or NIV and the level of PA (Table 1), although we should mention as a limitation of the study the fact that we did not know whether the patients had either a stationary or portable oxygen source.
It is known that air trapping has a limiting role in PA of COPD patients due to dynamic pulmonary hyperinflation31 and that daily PA in COPD patients is associated with it.32 In advanced COPD, dynamic hyperinflation during exercise is associated with greater dyspnea and poorer quality of life.33 Another limitation of our study is the incapability to collect this information in our patients.
One of the main features that characterizes COPD is the gradual deterioration in lung function, accentuated by exacerbations, which have been demonstrated to be a predictive factor for mortality in COPD,34 but the symptoms, functional status, measurements of the state of health and exercise capacity do not flow in a parallel manner. The complex varieties of cell, organic, functional and clinical manifestations that take place during COPD have increased the interest in unraveling the phenotypic heterogeneity of this disease.35 Likewise, we also know that performing PA is the result of a complex interaction of many factors, and that its behavior can only be analyzed from a multidimensional standpoint, in which in addition to disease severity other variables are taken into consideration, such as psychosocial and physical conditions and even cultural aspects. In fact, in our study the most inactive patients were those who lived with family members, who also had a sedentary occupation and whose income level was low. These data should stimulate us to develop more studies that help us to predict the start of the inactivity of our patients and to determine which indicators can alert us before its onset.
In the meantime, and in concluding, our study has allowed us to more thoroughly understand the habits of PA in the daily life of patients with stable COPD outside a respiratory rehabilitation program who are seen in pulmonology outpatient consultations. In these patients, there is evidence of low PA levels, which are associated with a poorer state of health and with greater disease severity.
Please cite this article as: Marín Royo M, et al. Actividad física y su relación con el estado de salud en pacientes EPOC estables. Arch Bronconeumol. 2011;47:335–42.