The 2017 edition of the Spanish Chronic Obstructive Pulmonary Disease Guidelines (GesEPOC) establishes a treatment algorithm based on the stratification of patient risk at 2 levels, low risk and high risk, according to three criteria: lung function, dyspnea grade, and history of exacerbations1. This stratification was initially based on a theoretical framework designed to determine the probability of the patient presenting an unfavorable clinical course. Since the publication of the algorithm, several studies have evaluated its prognostic capacity and endorsed its usefulness in this respect2,3.
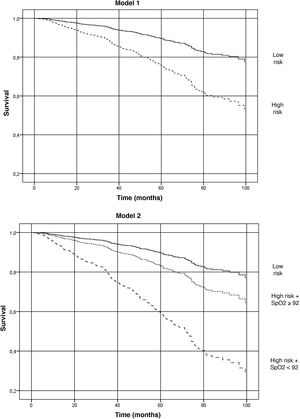
Chronic respiratory failure is associated with a higher mortality rate in patients with chronic obstructive pulmonary disease (COPD)4. Peripheral oxygen saturation (SpO2) is easy to determine by pulse oximetry, and values <92% correlate with the presence of severe hypoxemia5,6. On this premise, we conducted a retrospective study of patients with stable COPD monitored in a pulmonology clinic to analyze whether including the SpO2 measurement in the conventional GesEPOC risk criteria would improve the prognostic capacity of the algorithm. Consecutive patients with a diagnosis of COPD7 and a history of smoking (cumulative consumption >10 pack-years) were included. The following variables obtained at the first visit were recorded: lung function, body mass index (BMI), mMRC dyspnea grade, resting SpO2 (determined by the physician in clinically stable patients, breathing room air, at rest, and prior to physical examination), and history of exacerbations prior to inclusion, taking into account both moderate (requiring outpatient treatment with antibiotics and/or steroids) and severe (requiring emergency or hospital admission) exacerbations. Patients were classified as high risk and low risk according to the current GesEPOC criteria1 (in the presence of any of the following criteria the patient would be categorized as high risk: FEV1% <50%, dyspnea ≥2 if treated, 2 or more moderate exacerbations or ≥ 1 severe exacerbations the previous year), and this was compared with a classification in which high-risk patients were categorized as SpO2 <92% (high risk-SpO2 <92%) and SpO2 ≥92% (high risk-SpO2 ≥92%). A Cox regression survival analysis was performed to compare the two classifications: model 1 was obtained with the conventional GesEPOC classification; and model 2 included the variable SpO2 in the classification. The models were adjusted for age and comorbidity measured with the unadjusted Charlson index. The Akaike information criterion (AIC) was obtained for model comparison. Adjusted survival curves were obtained. Data collection was approved by the Santiago-Lugo Research Ethics Committee.
Overall, 710 patients were included, of whom 632 were men (89%), with a mean age of 68.3 ± 9.6 years, post-bronchodilator FEV1% 50.5 ± 17.2, and BMI 28.3 ± 5.2 kg/m2. One quarter (25.9%) were active smokers and the pack-year index was 59.3 ± 30.7. The Charlson comorbidities index was 1.98 ± 1.41. In total, 522 patients were classified as high risk (73.5%) and 188 (26.5%) as low risk. The mean SpO2 was 93.1 ± 4.6%. One hundred and sixty-four (23.1%) had SpO2 <92%, of which 155 were high risk. Mean follow-up time was 53.9 ± 26.7 months.
Overall mortality during follow-up was 25.8%, with significant differences between the low-risk group and the high risk group (10.1% vs 31.5%: P < .0001, respectively). The high risk-SpO2 group <92% had a mortality rate of 54.2%, compared with 21.9% in the high risk-SpO2 group ≥92% (P < .0001). In the Cox regression analysis, compared to the low-risk group, high-risk patients with SpO2 <92% have a greater risk of mortality (HR: 4.79; 95% CI: 2.90−7.91; P < .001) than high-risk patients with SpO2 ≥92 (HR: 1.7; 95% CI: 1.02−2.80; P < .001) (Table 1; model 2) (Fig. 1).
Results of logistic regression analysis based on the conventional GesEPOC classification (model 1) or with the inclusion of peripheral oxygen saturation (model 2).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Classification according to conventional GesEPOC criteria | ||||
| Low risk (reference) | ||||
| High risk | 2.53 (1.57−4.08) | <.001 | ||
| Classification according to conventional GesEPOC criteria + SpO2 | ||||
| Low risk (reference) | ||||
| High risk + SpO2 ≥92% | 1.7 (1.02−2.80) | <.001 | ||
| High risk + SpO2 <92% | 4.79 (2.90−7.91) | <.001 | ||
| Charlson comorbidity index | 1.31 (1.21−1.42) | <.001 | 1.31 (1.21−1.43) | <.001 |
| Age | 1.04 (1.2−1.06) | <.001 | 1.04 (1.02−1.06) | <.001 |
95% CI: 95% confidence interval; SpO2: peripheral oxygen saturation determined by pulse oximetry. Model 1 was analyzed using the conventional GesEPOC classification of high and low risk. In model 2, high-risk patients were classified according to their SpO2 value. Both models were adjusted for age and Charlson comorbidity index. The AIC for model 1 is 2,194.44 and for model 2, it is 2,063.95.
In our study, the addition of the SpO2 variable to the conventional GesEPOC criteria improves its prognostic capacity and classifies patients more precisely in terms of their mortality risk.
Factors, such as impaired ventilation-perfusion, dysregulation of respiratory center drive, or inspiratory muscle dysfunction due to pulmonary hyperinflation, contribute to the development of hypoxemia in COPD patients, impacting negatively on their life expectancy8–11. A cut-off point of SpO2 ≥92% is highly sensitive for ruling out respiratory failure, while SpO2 ≤88% is highly specific for confirming it5,6. Taking a pragmatic approach, and to avoid excluding any patient with respiratory failure, we selected a value of SpO2 <92%5. Thus, not all patients would meet the criteria for initiating long-term oxygen therapy (LTOT), so an intense bronchodilator therapy, sustained smoking abstention, and early onset of respiratory rehabilitation would hypothetically improve baseline SpO2 values12–15 and consequently, life expectancy. In line with the concept of personalized treatment proposed by GesEPOC, our approach may of particular interest in high-risk individuals with SpO2 <92% in whom the recommended initiation therapy would be a long-acting β2-agonist inhaled corticosteroid, as would correspond to the COPD-asthma phenotype. These subjects may benefit from early initiation of dual bronchodilation, despite the clinical impact that could be achieved with an inhaled corticosteroid. Furthermore, although this was not an objective of the study, we must point out that 9 patients in the low-risk group had SpO2 <92%. While this accounted for a very low percentage of patients, we believe that further studies that characterize these individuals in more depth may be needed to assess the clinical relevance of this finding.
Our study has limitations and essentially serves to generate debate. It is a retrospective study, conducted in a single center. The number of low-risk patients was very low, as SpO2 could not be used as a variable in these subjects. We cannot rule out that many of these patients were prescribed LTOT during follow-up, which could have influenced the reported mortality figures. Patients could not be stratified according to SpO2 ranges to avoid reducing statistical power. Despite these limitations, we believe that our results could be a stimulus for carefully designed studies that assess the inclusion of SpO2 in the conventional criteria for high-risk patients.
FundingNo funding was received for this manuscript.
Conflict of interestsThe authors declare that they have no conflict of interests directly or indirectly related with the contents of this manuscript.
Please cite this article as: Figueira Gonçalves JM, Golpe R, Ramallo Y, García Talavera I, Dacal D. ¿Debe incluirse la Saturación basal de oxígeno en la estratificación de riesgo de la enfermedad pulmonar obstructiva crónica propuesta por GesEPOC? Arch Bronconeumol. 2021;57:774–776.