Treatment with biological therapies increases the incidence of tuberculous disease. The introduction of systematic screening for latent tuberculosis infection in patients who are to receive these therapies has reduced this risk. In 2016, the consensus document on the prevention and treatment of tuberculosis in patients who are candidates for biological treatment was published in Spain. The main objective of this study was to evaluate adherence to these guidelines.
MethodsMulticenter, descriptive, observational study via an anonymous online survey sent to medical societies involved in biologics.
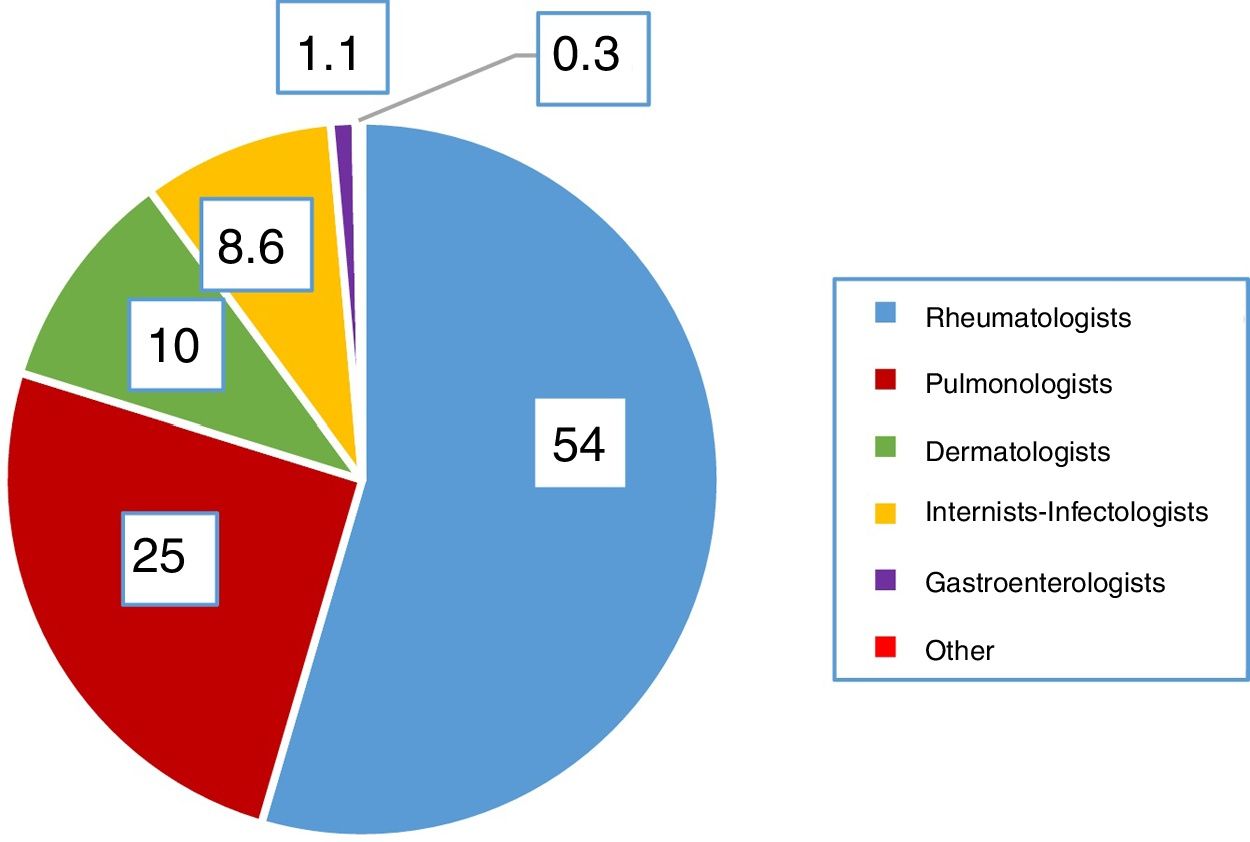
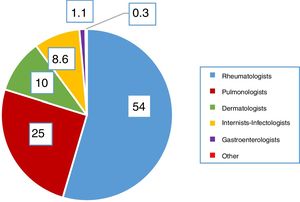
ResultsWe received 747 responses. Most respondents performed screening at the right time in the right patients (93.7%). Only 36.6% of respondents requested the appropriate diagnostic test, while 56.3% correctly recommended chemoprophylaxis. Up to 96% were familiar with the recommended chemoprophylaxis regimens, while only 63.9% initiated them at the right time. The specialist area that participated most and screened most patients for latent tuberculosis infection was rheumatology (54%). In most cases, pulmonologists were involved in an advisory capacity.
ConclusionsThis study shows poor overall adherence to recommendations, with only 56% of respondents reporting appropriate compliance. The incidence of tuberculous disease in patients who are to receive biological therapies could be reduced further by emphasizing the importance of the right diagnostic test and use of the diagnostic algorithm for latent tuberculosis infection.
El tratamiento con terapias biológicas aumenta la incidencia de enfermedad tuberculosa. La implementación sistemática del cribado de la infección tuberculosa latente en pacientes que van a recibir estas terapias ha conseguido reducir el riesgo de desarrollarla. En 2016 se publicó en España el Documento de consenso sobre la prevención y el tratamiento de la tuberculosis en pacientes candidatos a tratamiento biológico. El objetivo principal del estudio fue evaluar la adherencia al mismo.
MétodosEstudio multicéntrico, descriptivo, observacional en forma de encuesta anónima online, difundida entre las diferentes sociedades médicas que trabajan con biológicos.
ResultadosSe recibieron 747 respuestas. La mayoría de los encuestados realizaba el cribado en el momento adecuado y con la indicación correcta (93,7%). Solo un 36,6% de los encuestados solicitaba las pruebas diagnósticas adecuadas, mientras que el 56,3% acertaron las indicaciones de quimioprofilaxis. Hasta el 96% conocía las pautas de quimioprofilaxis recomendadas, mientras que solo el 63,9% las iniciaba en el momento adecuado. La especialidad con más participación y que más realizaba el cribado de infección tuberculosa latente fue reumatología (54%). En la mayoría de los casos, los neumólogos participaban como consultores.
ConclusionesEste estudio pone de manifiesto un bajo grado de adherencia a las recomendaciones, realizando un cumplimiento aceptable el 56% de los encuestados. Enfatizando en las pruebas diagnósticas adecuadas y en el algoritmo diagnóstico de infección tuberculosa latente, se podría reducir aún más la incidencia de enfermedad tuberculosa en los pacientes que van a recibir terapias biológicas.
The emergence of tuberculosis (TB) among patients who start biologics for the treatment of immune-mediated inflammatory diseases has alerted the medical community to the risk of reactivated TB infection associated with these therapies.1,2 The relative risk of TB in patients receiving these treatments has increased between 1.6- and 25-fold in recent decades,2–7 and the agents specifically associated with an increased risk are the anti-tumor necrosis factor-alphas infliximab and adalimumab. Two North American studies observed increased incidences of TB in patients with rheumatoid arthritis treated with infliximab (52.2–54 cases per 100000 patients per year) compared to those who did not receive biologics (6.2 cases per 100000 patients per year).4,8–10 The French RATIO registry recorded an incidence of TB among patients receiving infliximab of 116.7 cases per 100000 patients/year, 12.2-fold higher than in the general population.11 In Spain, Carmona et al. described a fourfold increase in the risk of TB among rheumatoid arthritis patients receiving biologics, compared with the general population.12 In Spain, more than 10% of candidates for biologics have latent TB infection (LTI), illustrating the scale of the population at risk of reactivated TB.13
In view of this evidence, international medical societies included LTI screening in their protocols as a requirement before starting biologics.14 This intervention has led to a 78–90% decrease in TB, although no studies have formally evaluated adherence to these recommendations. The Consensus Document on the Prevention and Treatment of Tuberculosis in Patients who are Candidates for Biological Treatment was published in Spain in 2016.15–17
The Emergent Group of the Tuberculosis and Respiratory Infections Study Area of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) conducted this study in collaboration with various medical societies, in order to evaluate the degree of adherence to the recommendations of the national consensus document.
Materials and methodsStudy designThis was a multicenter, descriptive, observational study based on an anonymous online Surveymonkey® questionnaire. Six scientific societies (Dermatology, Rheumatology, Digestive Diseases, Internal Medicine, Infectious Diseases, and Pulmonology) were invited to participate. The medical societies were contacted directly by email via their web site, and each invited their respective members to participate, using the online survey link.
Study objectivesThe primary objective was to determine adherence to the consensus recommendations. Secondary objectives were to identify which specialists performed LTI screening, to determine if screening was carried out in the respondent's center and to identify heterogeneity in LTI management, recommendations with a lower degree of uptake, the most widely used chemoprophylaxis, and the role of the respiratory medicine department in the process.
Data collectionThe survey consisted of 10 questions with 5 possible answers, some of which included an open-ended response (Table 1). It was not a prerequisite to be responsible for decision-making in this type of patient in order to participate in the survey. Indeed, since the responsibility and engagement of specialists varies among centers, we were interested to determine whether the professionals who might be involved at any stage of the process (whether prescribing biologics, seeing patients receiving these treatments, or managing tuberculosis) would know what to do. Respondents were not obliged to answer all the questions, and in some cases two answers were possible. Questions 1–4 collected general data, and questions 5–10 referred to the LTI screening consensus recommendations and chemoprophylaxis.
Screening for latent tuberculosis infection in patients who are candidates for biological therapies in Spain: survey questions.
| Question 1 What is your specialty? |
| 1. Respiratory medicine 2. Internal medicine/infectious diseases 3. Rheumatology 4. Dermatology 5. Gastrointestinal |
| Question 2 Do you screen candidates for biological therapies for latent tuberculosis infection in your hospital? |
| 1. Yes 2. No 3. Don’t know |
| Question 3 Which hospital departments carry out latent tuberculosis infection screening in candidates for biological therapies? |
| 1. Respiratory medicine 2. Rheumatology 3. Dermatology 4. Internal medicine/infectious diseases 5. Gastrointestinal |
| Question 4 What is the role of the respiratory medicine departments in latent tuberculosis infection screening in candidates for biological therapies? |
| 1. None 2. They supervise the process 3. They give advice if doubts arise 4. They are responsible for the whole process 5. The patient is referred to Respiratory medicine only if screening is positive |
| Question 5 Which candidates for biological therapies undergo latent tuberculosis infection screening? |
| 1. Latent tuberculosis infection screening is not initially performed 2. Screening is performed in patients with a history of previous exposure to tuberculosis 3. Screening is only performed when the patient develops symptoms suggestive of TB after starting biological therapy 4. Latent tuberculosis infection screening is performed in all patients who are candidates for biological therapies, always before starting treatment 5. Latent tuberculosis infection screening is performed in all patients who are candidates for biological therapies, but not always before starting treatment |
| Question 6 What tests do you request for latent tuberculosis infection screening? |
| 1. Chest X-ray+TT 2. Chest X-ray+IGRA 3. Chest X-ray+TT+IGRA 4. Chest X-ray+either TT or IGRA 5. Chest X-ray+TT. If TT is negative, I request IGRA |
| Question 7 If the TT is negative, what do you do next? |
| 1. I don’t need to do anything else, treatment with biologics can begin. 2. Repeat the TT in 2 weeks due to the booster effect 3. Request IGRA before starting treatment with biologics 4. I would start treatment with biologics, but I would monitor the patient with annual TTs during this treatment 5. I would request IGRA before starting treatment with biologics only if the patient is immunosuppressed or has recently received corticosteroids |
| Question 8 If the TT is positive, what do you do next? |
| 1. Repeat it in 2 weeks, in case it was a false-positive 2. Request IGRA to confirm the result 3. I would administer chemoprophylaxis for at least 2 weeks before starting treatment with biologics, after ruling out active TB 4. I would start chemoprophylaxis and biologics, after ruling out active TB. 5. I would administer chemoprophylaxis for at least 4 weeks before starting treatment with biologics, after ruling out active TB |
| Question 9 Is there any situation in which you would start chemoprophylaxis in a candidate for biologics with a negative TT and IGRA? |
| 1. No, it is not indicated 2. Yes, in immunosuppressed patients or those who have recently received corticosteroids 3. Yes, if there is evidence of untreated TB on the chest X-ray 4. Yes, if there is epidemiological evidence of recent exposure to TB 5. Yes, if either of the two previous options are applicable |
| Question 10 If chemoprophylaxis is prescribed, what regimen do you use most often? Note treatment duration in comments |
| 1. Isoniazid alone 2. Isoniazid with rifapentine 3. Rifampicin alone 4. Isoniazid with rifampin 5. I do not prescribe chemoprophylaxis; in this case, I refer the patient. |
The correct answers are indicated in bold.
Acceptable adherence to the recommendations was taken as correct responses to four or more of questions 5–10 (at least 60% correct answers). In contrast, low adherence was taken as incorrect responses to three or more of these questions (less than 50% correct). Scores were established according to the recently published “Health-Care Quality Standards in Chronic Obstructive Pulmonary Disease”,18 which propose a methodology for quantifying the implementation of recommendations.
Statistical analysisWe used the SAS statistical program 9.3 (SAS Institute, Cary, NC, USA). Qualitative data were described as absolute frequencies and percentages, and quantitative data were reported by mean, median, and standard deviation (minimum, maximum). The number of questions 5–10 with correct responses was calculated. Comparisons between two groups were performed using the Mann–Whitney U-test, and between more than two groups using the Kruskal–Wallis test. Other qualitative variables were compared using the χ2-test or the Fisher's exact test. All statistical tests were two-tailed, and values with p<0.05 were considered significant.
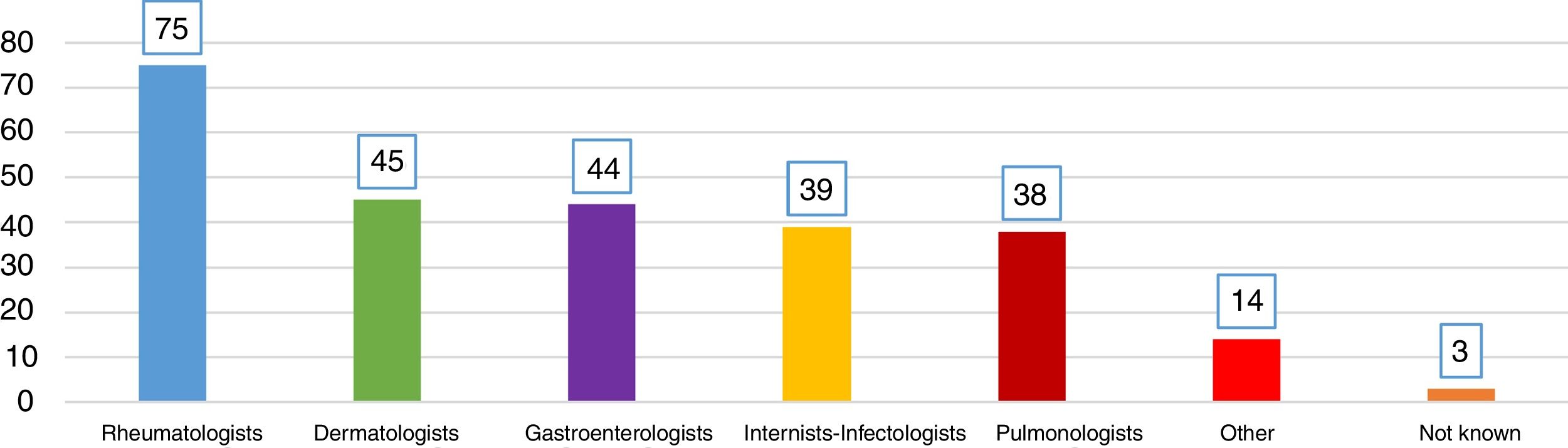
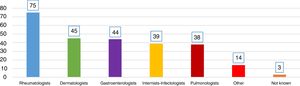
ResultsQuestions 1–4A total of 747 respondents completed the survey. Of these, 62% of the respondents were women and their mean age was 46 (±10.6) years (25–81 years). In 98.1% of cases, LTI screening was performed in the respondent's hospital. Participation by specialties is shown in Fig. 1. Highest participation was among rheumatology specialists (54%), and this group performed the most screening. Fig. 2 shows which specialists performed screening in each center.
The role of the pulmonologist in LTI screening is advisory in 43% of cases, in 38% they do not participate, in 8% they conduct the full study, in 3% of cases they supervise the process, and in 18% of cases they only see referrals with positive screening.
Questions 5–10With regard to which biological therapy candidates should undergo LTI screening and when, 93.7% of respondents replied that they all should (correct answer). Another 2.8% perform screening, but not always before starting treatment; 1.6% do not screen any patients; 0.4% screen patients only if they develop clinical symptoms suggestive of TB after starting biologics; 1.2% only screen patients with a history of contact with TB patients.
With regard to which tests should be requested for LTI screening, 36.6% of respondents selected simultaneous performance of chest X-ray, interferon gamma release assay (IGRA), and tuberculin test (TT) (correct answer). A total of 45.6% stated that they only performed chest X-ray and TT, and 11.4% performed chest X-ray and IGRA. The remaining 9% performed chest X-ray, and either TT or IGRA, but not both, even if they were negative.
With regard to the action to be taken in the case of negative TT, only 26.1% of respondents stated that they requested IGRA before starting biologics (correct answer); 57.6% would repeat TT after 2 weeks due to the booster effect; 9.4% would only request IGRA in immunosuppressed patients or those who had received corticosteroids. A total of 6.9% would not perform more tests and would initiate biologics at that time, although almost half of them (3.7%) would subsequently monitor their patients with annual TT during the biologics treatment period.
In response to the question on what to do in the case of positive TT, 63.9% of respondents replied that they would prescribe 4 weeks of chemoprophylaxis before starting biologics (correct answer); 15.4% would administer chemoprophylaxis for only 2 weeks before starting biologics; 8.7% would begin chemoprophylaxis and biologics simultaneously, after ruling out active TB; 0.8% would repeat TT in 2 weeks, in the case of a false-positive; and 11.2% would request IGRA to confirm the result.
In response to the question on whether in some cases they would start chemoprophylaxis if TT and IGRA were negative, 56.3% of the respondents replied that they would prescribe chemoprophylaxis if there was evidence of untreated TB on the chest X-ray or epidemiological evidence of recent exposure to TB (correct answer). In total, 19.1% would start chemoprophylaxis only if there was evidence of untreated TB on the chest X-ray; 13.6% would do so if there was evidence of recent exposure to TB; 3.6% would administer chemoprophylaxis to immunosuppressed patients or those who had recently received corticosteroids. In contrast, 17.4% would not start chemoprophylaxis in any of these cases, believing that it was not indicated.
Finally, when asked about chemoprophylaxis regimens, 96% replied correctly; most respondents would prescribe either isoniazid (H) in monotherapy (83%) or combined H and rifampicin (R) in special cases. The treatment duration proposed by most respondents (9 and 3 months, respectively) complied with the national consensus. Of the remaining respondents, 1.5% used monotherapy with R, 1.9% used H with pyrazinamide, and the same percentage used H with rifapentine.
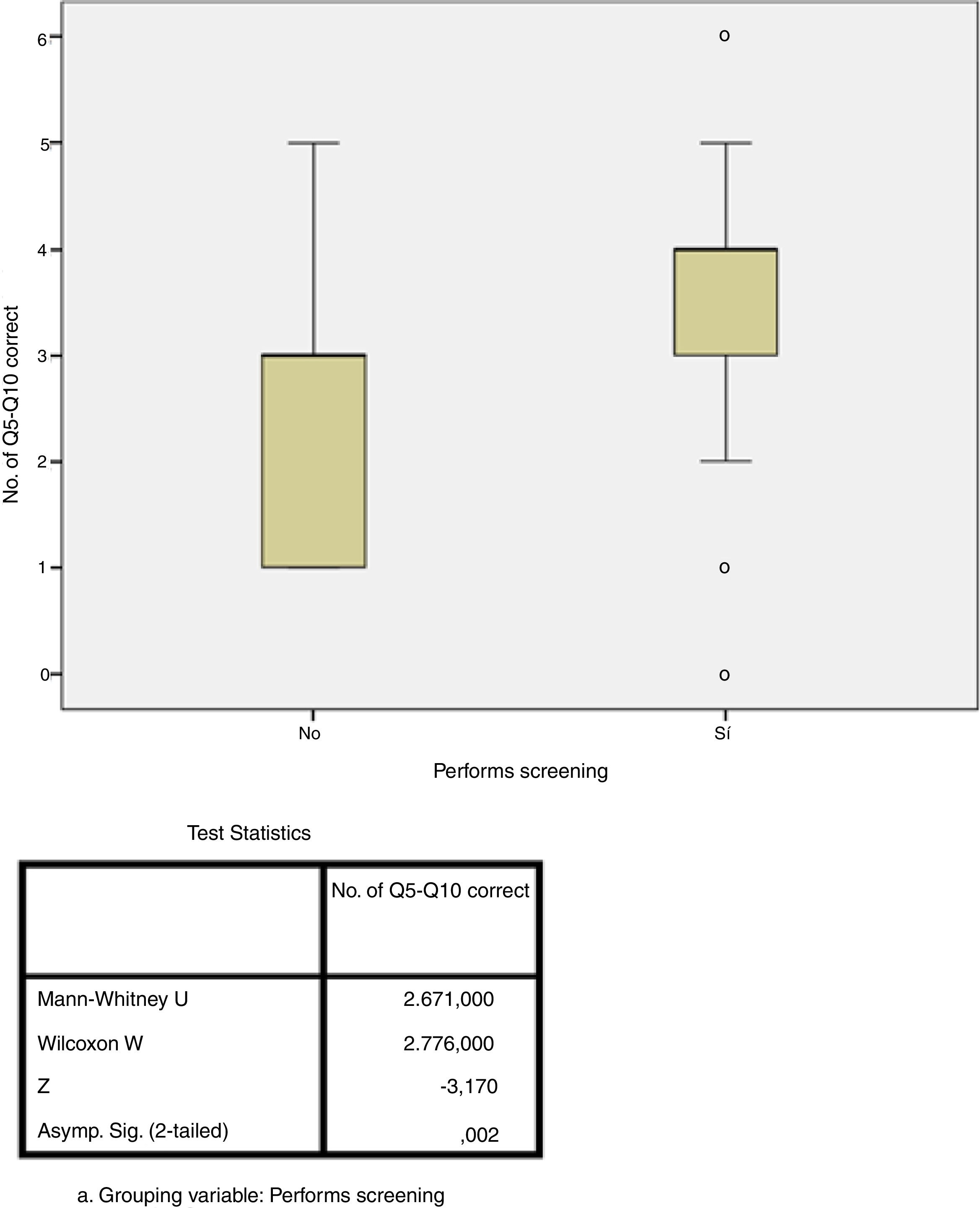
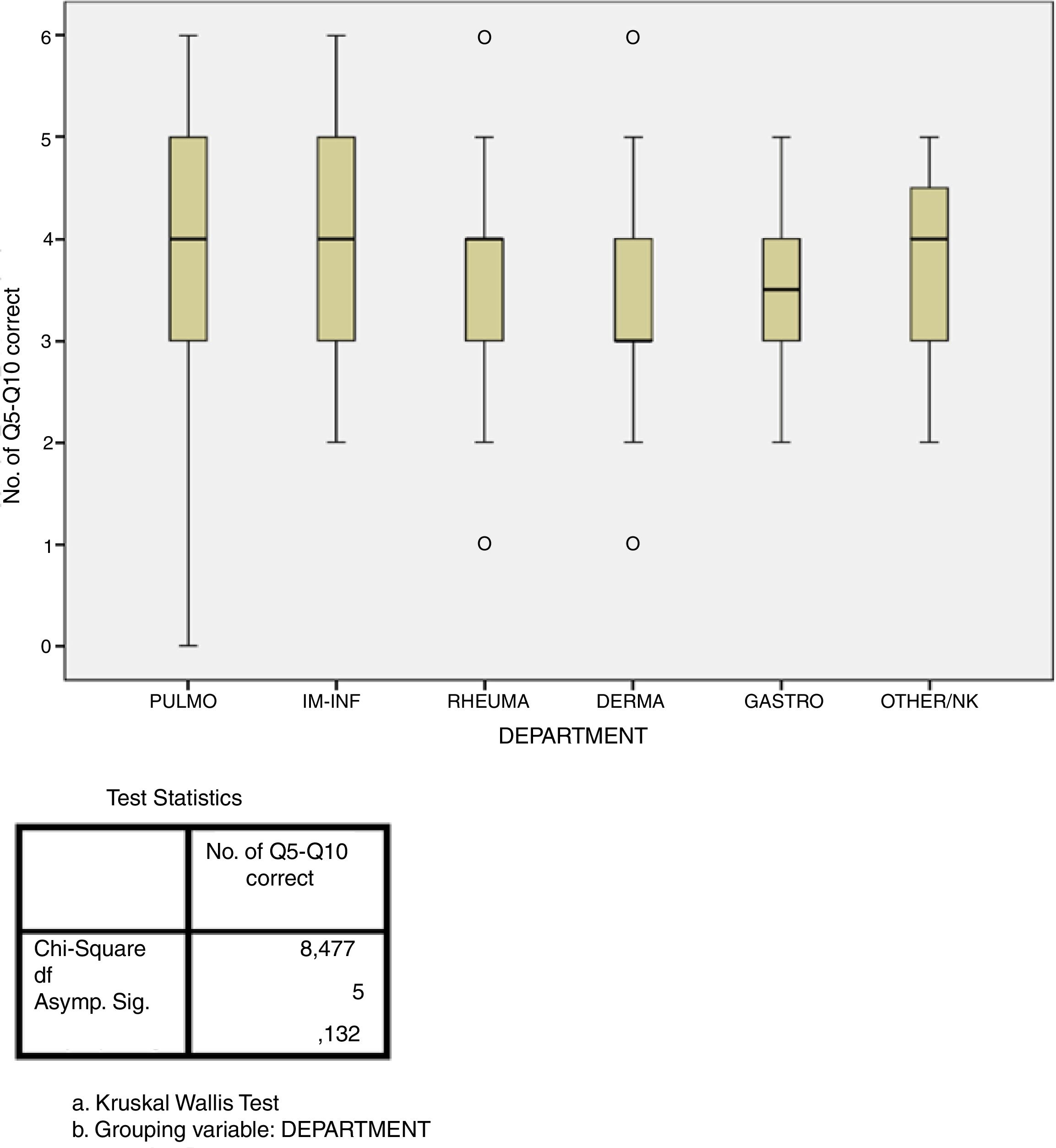
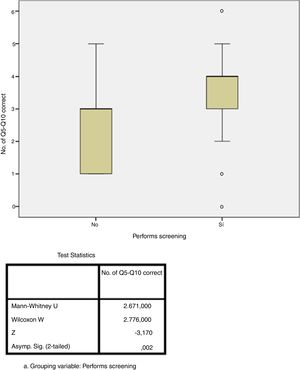
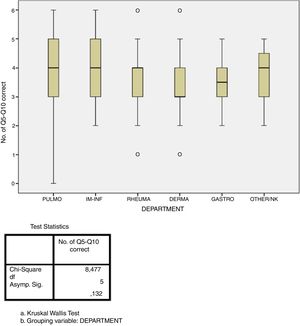
Adherence studyThe answers of 56% of respondents revealed adherence of at least 60% to the recommendations of the national consensus (correct responses to at least four of the six questions), although only 5.6% answered all six questions correctly. In contrast, 43% of respondents had a low percentage of correct answers (correct responses to three or fewer of the six questions). The performance of LTI screening in the respondent's own hospital was the only factor that showed a statistically significant association with greater adherence (p=0.002, Fig. 3). There were no significant differences in the degree of adherence according to medical specialty (p=0.132, Fig. 4). The rate of adherence by specialty is shown in Fig. 4.
Questions addressing in whom and when to perform LTI screening, and chemoprophylaxis regimens and duration, were answered correctly by 93 and 94% of respondents, respectively. In contrast, questions concerning the action to be taken in the case of negative TT and the tests that should be requested for screening were only answered correctly by 25 and 36% of respondents, respectively.
DiscussionThis survey of a wide range of medical specialists involved in the administration of biologics revealed a low degree of adherence to the recommendations of the Spanish Consensus Document on the Prevention and Treatment of Tuberculosis in Patients who are Candidates for Biological Treatment.16 Only 56% of respondents showed acceptable adherence to the recommendations. In 98% of cases, LTI screening was performed in the respondent's own hospital, and this was the only variable associated with better adherence to the consensus. The percentage of respondents who conduct the screening themselves and their role in the process is unknown. It can therefore be assumed that specialists who replied voluntarily to the survey participate directly or indirectly in LTI screening, or at least are aware of it as part of their training or clinical practice as a specialist involved either in the treatment of TB or the administration of biologics. Most respondents performed screening at the right time in the right patients. However, only 36% requested the right diagnostic tests and did not include the determination of IGRA in their routine practice. The questions that were most often answered correctly focus on when to begin chemoprophylaxis and which regimens to use. Nevertheless, it was striking to observe that almost 24% of respondents would not start chemoprophylaxis with a positive TT, and almost 20% would not prescribe it in any case with negative TT or IGRA, despite recent exposure to TB and/or immunosuppression.
These data are similar to those of the Spanish registry of systemic treatments in psoriasis (BIOBADADERM), recently published by Sánchez Moya et al.,17 which included more than 1400 patients with moderate–severe psoriasis, with an estimated LTI prevalence of 20.5%. Screening was conducted in 83% of patients, but only 51% adhered to the recommendations of the consensus document. No other studies have published on adherence to LTI recommendations.
Although all experts agree that the incidence of TB is greater at the beginning of biological therapy, discrepancies emerge regarding the minimum duration of chemoprophylaxis before the administration of biologics can safely begin. The initial guidelines recommended at least 1 month, but this interval was reduced to 3 weeks after publication of the study by Carmona et al.11 Several recent studies, including the American recommendations of the Centers of Disease Control and Prevention, even suggest that both treatments can safely be started simultaneously without waiting.19,20 However, in Spain, the consensus document still recommends waiting 3 weeks, during which time proper chemoprophylaxis adherence and tolerance can be confirmed.15 According to the results of our study, most respondents (63.9%) follow this recommendation.
Evidence clearly supports the effectiveness of the recommendations after implementation in clinical practice.15,20,21 In Spain, data published by the Spanish registry of adverse events associated with biologics in rheumatic diseases (BIOBADASER) show a reduction of 74% in TB cases since 2002, the year in which LTI screening was introduced. However, estimates suggest that recommendations were not followed appropriately in up to 20% of cases, mainly due to the failure to repeat TT for the booster effect, leading to a sevenfold increase in the risk of TB.15,17,22 In the BIOBADASER registry, the incidence of TB was 20 times higher than that of the general population before LTI screening became generalized and fell by 78% after its introduction. Thus, the rate of TB among rheumatoid arthritis patients receiving biologics has fallen by 86%, achieving similar rates as among patients not receiving these agents.15
A closer look at the tests requested for LTI screening shows that the consensus document recommends that patients are actively screened for a personal history of TB, contact with patients with active TB, and old TB lesions on chest X-ray and that IGRA and TT are performed simultaneously. The high rate of false-positives in TT, due mainly to previous vaccination, but also to its low sensitivity in patients receiving immunosuppressive therapy (up to 40% of false-negatives in some series),21,23–27 support the recommendation to routinely perform both TT and IGRA in these patients. However, our survey results also reveal that IGRA testing is not fully integrated into routine clinical practice. Repeating TT to increase sensitivity due to the booster effect is another of the most frequent errors in the survey responses: this practice is not recommended in the consensus document, as it reduces the specificity of the test and increases the rate of false positives.28 The lack of adherence to the consensus recommendations regarding diagnostic tests revealed by the survey may be due to heterogeneity of the criteria specified by the different international medical societies.
According to the results of the survey, pulmonologists in most cases have an advisory role (43% of responses) or are involved in the management of TB. The respiratory medicine department is involved in screening in the centers of only 38% of the respondents. It could be assumed from these data that as pulmonologists do not routinely prescribe biologics, they see only a small number of candidates for biological therapies and that the specialists who do prescribe these drugs also perform LTI screening in their patients. Therefore, fewer than half of screening candidates will be seen in the respiratory medicine department. This may have been one of the reasons why 20% of the respondents reported that they would not start chemoprophylaxis in cases in whom it should be indicated, as these are specialists who have less experience in the management of TB.
The study has some limitations, mainly due to the anonymous online survey format, an approach which has methodological implications when it comes to drawing conclusions. The facilities available in each participating center (access to determination of IGRA, presence of pulmonologists, etc.) were not investigated, and this may have influenced some results. Nor do we know the total number of specialists contacted and, consequently, the overall proportion who responded. Furthermore, the overrepresentation of rheumatologists (54% of respondents) compared to the scant representation of specialties such as respiratory medicine, internal medicine, or infectious diseases may have biased the results of LTI management, since these are the three specialist departments that routinely see most patients of this type. Additionally, in this study, 98% of respondents stated that they perform LTI screening in their own hospitals, underlining the low degree of overall compliance.
Our study also has a number of strengths, including the fact that we collected the opinions of a broad sample of physicians who reported on their routine clinical practice, suggesting that the results are representative. Moreover, the questions were designed to allow a certain degree of variability, avoiding categorical responses or responses with only one possible answer, which also reflects real-world care in our setting.
ConclusionsIt is now 15 years since the introduction of biologics and 1 year since the publication of the national consensus document on LTI screening in candidates for biological therapy, yet 43% of respondents still do not follow the recommendations with an acceptable degree of adherence.
Most respondents, irrespective of their specialty, identify the right candidates and the right time for LTI screening before starting biologics and select the correct regimen and duration of chemoprophylaxis. However, only 36% requested the appropriate diagnostic tests and most failed to act appropriately when TT was negative, one of the responses with the lowest percentage of correct answers. This suggests that IGRA testing is underused in daily clinical practice, perhaps because access is limited in some centers or because physicians are unaware of it. The fact that some medical societies make recommendations that diverge from the national consensus may also influence matters. It seems likely that if the implementation of the recommended diagnostic algorithm is reinforced, the incidence of TB, that has fallen since systematic LTI was introduced, may be reduced even further.
Conflicts of interestThe authors state that they have no conflict of interests.
- -
Statistics Department of HGU La Paz de Madrid.
- -
Spanish Societies of Rheumatology (SER), Spanish Academy of Dermatology and Venereology (AEDV), Digestive Diseases (EDPS), Internal Medicine (SEMI), Infectious Diseases and Clinical Microbiology (SEIMC), and Pulmonology and Thoracic Surgery (SEPAR).
- -
SEPAR Tuberculosis Working Group.
- -
SEIMC Mycobacterial Infections Study Group.
- -
Coordinator of the SEPAR Area of Tuberculosis and Respiratory Infections (TIR).
- -
Coordinator of the SEPAR Tuberculosis Integrated Research Program.
Please cite this article as: Quirós S, de la Rosa D, Uranga A, Madero R, Amaro R, Bruguera N, et al. ¿Cómo realizamos el cribado de infección tuberculosa latente en pacientes candidatos a terapias biológicas en España? Una encuesta multidisciplinar. Arch Bronconeumol. 2018;54:510–517.