Atrophy and weakness of the respiratory and peripheral muscles is a common problem in the intensive care unit (ICU). It is difficult to diagnose, particularly in the early stages of critical disease. Consequently, many cases are detected only in advanced stages, for example, when difficulties in mechanical ventilation weaning are encountered. The aim of this review is to describe the main tools that are currently available for evaluation of peripheral and respiratory muscles in the ICU. Techniques of varying complexity and specificity are discussed, and particular emphasis is placed on those with greater relevance in daily clinical practice, such as ultrasound.
La atrofia y la debilidad de los músculos respiratorios y periféricos son un problema frecuente en las Unidades de Cuidados Intensivos (UCI). Su diagnóstico es dificultoso, especialmente en las primeras etapas de la enfermedad crítica. Esto determina que en muchos casos no sea detectada hasta etapas avanzadas, por ejemplo, frente a dificultad para la desvinculación de la asistencia ventilatoria mecánica. El objetivo de esta revisión es describir las principales herramientas con las que contamos actualmente para la evaluación muscular en la UCI, tanto periférica como respiratoria. Se mencionan técnicas con distinto grado de complejidad y especificidad, haciendo particular hincapié en aquellas con mayor aplicabilidad en la práctica clínica diaria, como la ecografía.
Many patients admitted to the intensive care unit (ICU) develop wasting and weakness of the peripheral and respiratory muscles.1,2 This impacts negatively on morbidity and mortality during hospitalization and after discharge, and is associated with difficulty in weaning from mechanical ventilation (MV), longer stays in the ICU, and increased short and long-term mortality.3–14
Myopathy is the product of a combination of contractile dysfunction and muscle atrophy, and its pathophysiology in the critically ill patient is complex.15 Contractile dysfunction (decreased intrinsic capacity of the muscle to produce force) may be caused by the combined action of a number of factors, such as mitochondrial dysfunction, oxidative stress, excitation-contraction disorders, and membrane potential.15 Wasting (decreased muscle mass and myofiber size) is the result of an imbalance between the synthesis and degradation of proteins, in favor of the latter, due mainly to activation of the ubiquitin–proteasome system.16 The clinical manifestations are, therefore, a result of decreased muscle mass and strength.
Diagnosis and follow-up of muscle impairment can be difficult in critically ill patients. The aim of this narrative review is to summarize the main methods available for the functional and structural assessment of the peripheral and respiratory muscles in the ICU.
Respiratory MusclesRespiratory muscles are routinely not assessed in general, so diagnosis of diaphragmatic dysfunction is delayed until difficulties are encountered in weaning the patient from MV.17,18 We will review below the main techniques for assessing respiratory muscles in critically ill patients.
Maximum Inspiratory PressureMaximum inspiratory pressure (MIP) provides a useful overall assessment of inspiratory force.19 MIP is the maximum negative pressure generated during an inspiratory effort against an occluded airway.20 This technique requires patient collaboration, so higher values rule out significant weakness, but low values may reflect muscle weakness, an inefficient technique, or inadequate effort.17 If the patient cannot cooperate, the maneuver can be carried out with a 1-way valve that allows expiration but not inspiration, obtaining MIP in a 20–25s period.17 Values of less than −30cmH2O show high sensitivity (but low specificity) for predicting failure to wean from MV.21
Another measurement, in addition to MIP, is sniff nasal inspiratory pressure (SNIP).19,22 SNIP consists of a voluntary inspiratory maneuver, in the form of a short, rapid inhalation through a patent nostril. SNIP is a simple and reproducible measurement for evaluating inspiratory muscle strength. Moreover, the sniff nasal maneuver can be used to assess muscle function in association with other techniques, such as transdiaphragmatic pressure (Pdi) or ultrasonography. In critically ill patients, the feasibility of the SNIP is usually determined by the presence of an artificial airway.
Esophageal And Transdiaphragmatic PressuresSimultaneous recording of esophageal (Pes) and gastric (Pga) pressure can be used to calculate Pdi (Pdi=Pes−Pga), a specific measurement of diaphragmatic force.17,19 Pdi can be measured during quiet breathing or during voluntary maximum inspiratory (Pdimax) or nasal sniff (Pdisn) maneuvers. The Gilbert index (ΔPga/ΔIdps) can be used to determine the contribution of the diaphragm to the total inspiratory effort (a higher score indicates greater contribution).19 Other parameters, such as the pressure–time product (PTP) and tension–time index (TTI) can be used to estimate the energy expenditure of the diaphragm, although this analysis is complex.17 PTP is the integration of respiratory pressure over time, normalized on the basis of maximum pressure in the case of TTI.23 Its use is generally reserved for the field of research.17
Twitch, the electrical or magnetic stimulation of the phrenic nerve, is a technique used to measure Pdimax without the collaboration of the patient (Pditw).19,22,24 In fact, Pditw determined by bilateral anterior phrenic nerve magnetic stimulation can be considered the gold standard for evaluating diaphragmatic strength in the ICU.19,24 As an alternative to Pdi, pressure recorded at the end of the tracheal tube after phrenic nerve stimulation (Ptrtw) can be used as a parameter of diaphragmatic strength, without the need for catheters for recording Pes and PGA.25 Values of PDItw<10cmH2O or Ptrtw<11cmH2O are parameters of diaphragmatic weakness in critical patients.6,7,19 Reduced PIM, Pditw, and PTRtw have been associated with difficulty in MV weaning and increased mortality.5–8,26
Occlusion PressureThe negative pressure generated during the first 100ms of a quiet inspiration against an occluded airway (P0.1) can be used to assess central ventilatory stimulus.19,20 High values of P0.1 indicate an increased central stimulus, while low values of P0.1 can be due not only to a reduced neuromuscular impulse, but also to nerve conduction problems, muscle weakness or fatigue.19 It has been used in patients receiving MV as a parameter for predicting success in weaning. P0.1 values ≥6cmH2O predict weaning failure, while P0.1≤4cmH2O is considered favorable.19 However, very low values (<0.5cmH2O) may represent a reduced central impulse that is insufficient to achieve weaning.20
Electrophysiological Studies: Electromyography And Stimulation TestElectromyography (EMG) is used to study muscular activity by recording action potentials.22 Diaphragmatic EMG can be obtained from surface, esophageal or intramuscular electrodes.19 Nasogastric feeding tubes with embedded esophageal electrodes (Maquet, Sweden) can be used to obtain a continuous processed signal called “diaphragmatic electrical activity” (EAdi).17 Stimulation tests can be used to assess the efficiency of nerve and neuromuscular transmission with diaphragmatic electromyographic recording after phrenic nerve stimulation.19
Diaphragm UltrasonographyDiaphragm ultrasonography, available in most ICUs, is a noninvasive technique that can be used to monitor diaphragm dysfunction at the patient's bedside. Ultrasonography can assess the diaphragmatic mass using static measures such as diaphragm thickness (Tdi), and functional aspects can be evaluated with proactive measures such as thickness fraction (TFdi) or diaphragmatic excursion (Edi).27,28
This technique can be used in most critical patients, and has excellent intra- and inter-observer reproducibility.27 Although ultrasound evaluation can be performed both in the right and left hemidiaphragms, the left side presents greater technical complexity, making it more difficult to visualize in certain patients and reducing the reproducibility of the test.29
Diaphragm ThicknessDetermining Tdi by ultrasound allows an assessment of the muscle mass that correlates very well with the direct measurement of muscle thickness on autopsy.30,31 Tdi is measured at the end of a passive expiration, that is to say, at functional residual capacity (FRC). In healthy adults, the average values of Tdi range between 1.7±0.4 and 3.3±1.0mm.30,32–36 In critically ill patients receiving MV, values of between 1.3±0.3 and 2.4±0.8mm have been reported.29,37
It is difficult to establish a cut-off point below which the diaphragm can be considered to be atrophied. However, performing serial ultrasounds after initiating MV can reveal an early, progressive decline in Tdi during the stay in the ICU.37–40 However, the determination of Tdi as a static measurement has not proved useful as a parameter for predicting success in weaning from MV.14,41–43
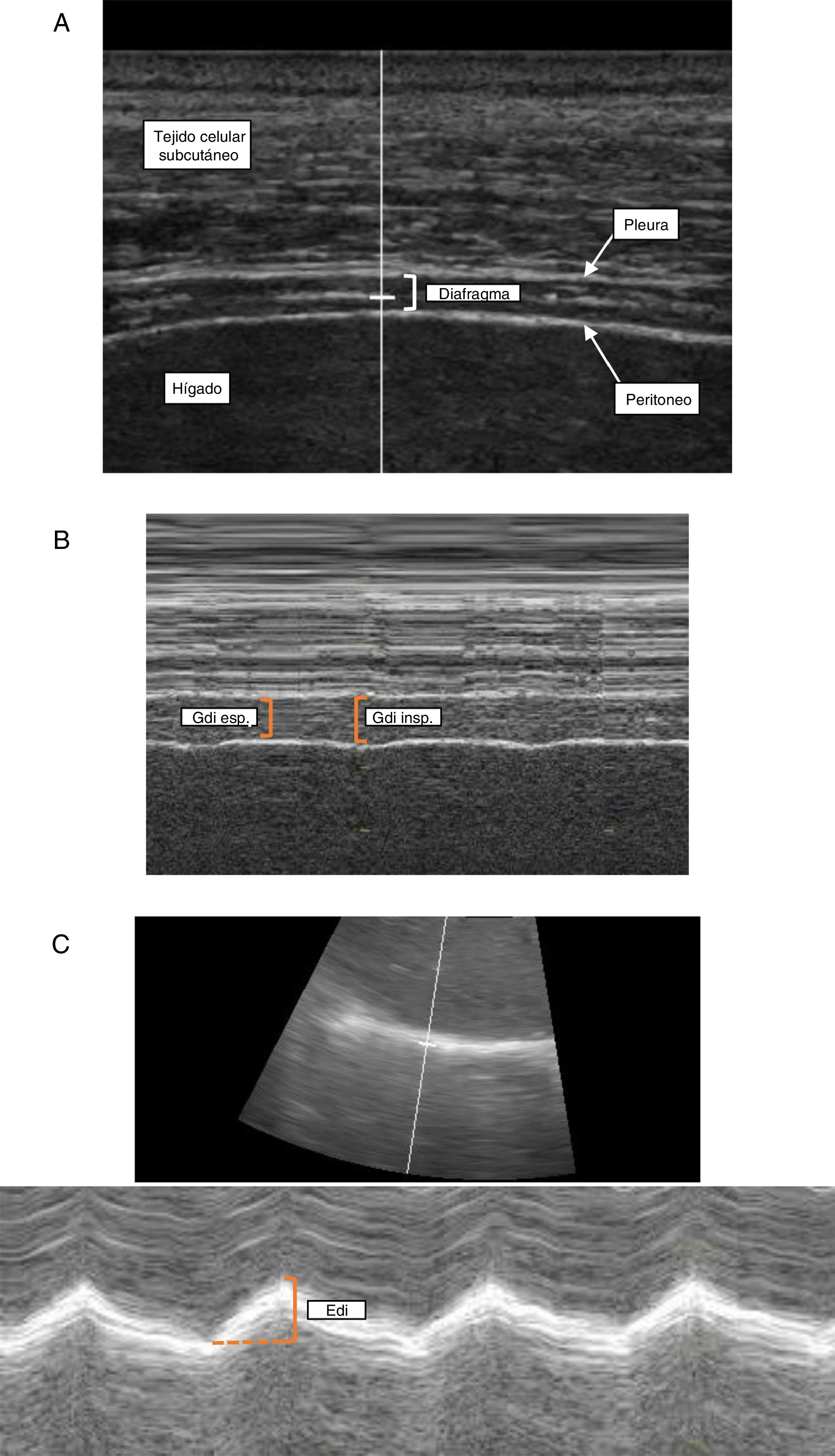
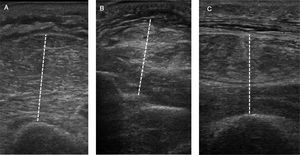
Technique. The measurement of Tdi with ultrasound is performed in the right hemidiaphragm at the level of the zone of apposition. The zone of apposition is the area of the chest wall where the abdominal contents meet the lower rib cage, between the 8th and 10th intercostal space, at the level of the mid axillary line. With the patient supine (30–45°), a high-frequency linear transducer (≥10MHz) is placed parallel to the intercostal space and perpendicular to the skin.27 It is first used in 2-dimensional mode (mode B) to obtain the best view and to select the scan line (Fig. 1A). In this area, the diaphragm is identified as a structure formed of 3 parallel layers (approximately 1.5–3.0cm deep): a hypoechoic central layer (the diaphragm itself) demarcated by 2 hyperechoic layers (the peritoneum and parietal pleura).44 The pulmonary artifact (corresponding to the costophrenic angle) can also be seen to erase the image of the diaphragm during inspiration.36 Keeping the transducer in position, M mode is used to determine Tdi, corresponding to the distance between both hyperechoic layers, measured approximately 2cm from the costophrenic angle (Fig. 1B). As mentioned previously, Tdi is measured at the end of the passive expiration phase. However, determining Tdi during different stages of the respiratory cycle is useful for evaluating functional parameters such as TFdi.
Diaphragmatic ultrasonography. Determination of diaphragm thickness in the zone of apposition with the chest wall in B-mode (A) and M-mode (B) and evaluation of diaphragmatic excursion (C). Edi: diaphragmatic excursion; Gdi esp: expiratory diaphragm thickness; Gdi insp inspiratory diaphragm thickness; Tejido celular subcutáneo: subcutaneous cellular tissue; Higado: liver; Diafragma: diaphragm; Pleura: pleura; Peritoneo: peritoneum.
During spontaneous ventilation, lung volume increases as the diaphragm contracts, shortens, and increases in thickness.30,45 Diaphragmatic thickening during inspiration is a parameter of the contractile activity of the diaphragm and its ability to generate pressure.27,40,45 This can be assessed on ultrasonography by measuring TFdi, which corresponds to the percentage of change in Tdi between the end of passive expiration and the end of inspiration (maximal inspiratory effort in spontaneous ventilation, or if that is not possible, inspiratory peak during MV).40
TFdi has shown good correlation with inspiratory muscle function parameters such as MIP, SNIP, Pdi, PTP and EAdi.29,34,42,45–47
In patients receiving MV, TFdi decreases progressively as the level of ventilatory support increases, becoming minimal in controlled ventilation with neuromuscular blockade.29,34,37 In other words, the more work of breathing generated by the diaphragm, the greater the TFdi. The same technique could be used to monitor diaphragmatic activity during MV.37 Goligher et al. showed that low levels of TFdi are associated with greater diaphragmatic wasting (decreased Tdi) in patients on MV.40 The presence of reduced TFdi has been associated with failure to extubate, difficulty in weaning from MV, and prolonged ICU stay.9,14,41 Although it is difficult to establish cut-off points, various studies have used TFdi values of between 20% and 36% to define the presence of diaphragmatic dysfunction.4,9,41,42
Technique. The determination of TFdi requires the measurement of Tdi at the end of the expiration phase and at the end of inspiration. In practical terms, this corresponds to minimum and maximum values, respectively, while measuring Tdi in M mode (Fig. 1B).27,29
Diaphragm ExcursionEdi is another dynamic parameter that can provide information about the diaphragm. It is determined by visualizing and measuring the cephalocaudal shift of the dome of the diaphragm between expiration and inspiration. In individuals with spontaneous ventilation, Edi can be assessed in quiet breathing, deep inspiration and with a sniff nasal maneuver (in all cases at FRC). Edi during a deep inspiration correlates with MIP, SNIP, forced vital capacity and forced expiratory volume in 1s.46,48 According to the largest series reported in healthy volunteers, Edi is somewhat greater in men than in women: 1.8±0.3cm vs 1.6±0.3cm in quiet breathing, and 7.0±1.1cm vs 5.7±1.0cm in deep inspiration, respectively.48 Similar values have been found in other studies.46,49,50 In spontaneous ventilation, a decreased range of motion indicates diaphragmatic weakness, while the absence of movement or paradoxical movement is an indicator of diaphragmatic paralysis.50,51 However, in patients receiving MV, Edi is a product not only of muscle contraction, but also of passive movement caused by ventilatory support.44 As such, it has not been possible to correlate Edi with other parameters of diaphragmatic function in patients receiving MV.47 However, Edi (less than 1.0–1.1cm in quiet breathing) has been shown to be useful for predicting the failure of extubation when performed in patients during a spontaneous ventilation trial.3,52,53
Technique. With the patient sitting at a 30–45° angle, a convex transducer (3.5–5MHz) is applied at the right subcostal level between the clavicular line and the anterior axillary line.27,48 The transducer is moved in a medial, cephalic and dorsal direction, so that the ultrasonic beam reaches the posterior third of hemidiaphragm in a perpendicular position.44 It is first used in mode B to obtain the best view and to select the scan line. In this mode, the diaphragm is identified as a hyperechoic line directly associated with the liver below and the pulmonary parenchyma above. Once the structures are identified in B-mode, the scan line is used in the most perpendicular position possible to the diaphragm and the M-mode is activated, in order to evaluate the movement of anatomical structures along the selected line (Fig. 1C). Edi will be measured as the diaphragmatic shift from the end of the expiration phase until the end of inspiration. This technique can also be used to determine other parameters, such as speed of contraction and relaxation (cm/s), inspiratory and expiratory times, and total duration of the cycle.27,49
Peripheral MusclesPeripheral muscle strength in the ICU can be studied with Medical Research Council (MRC) manual muscle testing or manual prehension dynamometry. Both methods require the collaboration of the patient, so their use during the early stages of critical disease is usually limited. It is at this stage that muscle impairment begins to develop, so other methods are needed for early detection.54 In this respect, electrophysiology and ultrasonography may provide information on structural and functional alterations before the weakness becomes clinically evident.
Manual Muscle TestingThe overall assessment of strength using MRC manual muscle testing is currently the gold standard for the diagnosis of ICU-acquired weakness (ICUAW).55 The MRC scale evaluates the strength of 3 muscle groups in each upper and lower limb, assigning a value to each muscle group of between 0 (paralysis) and 5 (normal strength), and a total value of 0 to 60 (Table 1).56 The evaluation is carried out using certain standardized maneuvers (Table 2).57
MRC Scale for Manual Muscle Testing.
| Grade 0 | No visible or palpable contraction |
| Grade 1 | Flicker or trace of contraction, no limb movement |
| Grade 2 | Movement with gravity eliminated |
| Grade 3 | Movement against gravity over almost full range of motion |
| Grade 4 | Movement against moderate resistance |
| Grade 5 | Movement against complete resistance (normal power) |
MRC: Medical Research Council.
Manual Muscle Assessment Protocol (MRC) in the ICU.
| Movement | Procedure |
|---|---|
| Shoulder abduction | Lift the outstretched arm out from side at shoulder level. Place a hand above the elbow and the other on the shoulder, apply resistance (grade 3, 4 or 5). If grade <3, place the patient in a supine position, support the arm above the elbow and at the wrist and ask them to move it out to the side. Grade 2 is assigned if the patient moves it with gravity eliminated. If they cannot move it, palpate the deltoid muscle to distinguish between grade 1 and 0. |
| Elbow flexion | Forearm supinated and flexed >90°. Place a hand above the wrist and the other on the shoulder, apply resistance (grade 3, 4 or 5). If grade <3, abduct the shoulder to 90° with the arm outstretched and thumb pointing up, holding it at the height of the elbow and wrist and ask the patient to flex it. Grade 2 is assigned if they can move it with gravity eliminated. If they cannot move it, position the arm at the side, with the forearm supinated, in 45° of elbow flexion, and palpate the biceps tendon to discriminate between grade 1 and 0. |
| Wrist extension | Arm at the side with the elbow flexed to 90°, forearm pronated and hand extended (support the elbow). Ask the patient to extend their hand as far as possible. Place one hand on the patient's hand, distal to the wrist, the other hand holding the forearm. Apply resistance (grade 3, 4 or 5). If grade <3, the elbow is flexed to 90° with the thumb pointing upwards. Support the elbow and wrist of the patient, and ask them to extend their hand. Grade 2 is assigned if they can extend it with gravity eliminated. If they cannot move it, in the same position palpate the extensor tendons in the wrist to discriminate between grade 1 and 0. |
| Hip flexion | Flex the hip with the knee bent. Place a hand above the knee and the other on the hip, apply resistance (grade 3, 4 or 5). If grade <3, the patient should lie down on the side not being tested. Support the leg with one hand below the knee, maintaining alignment with the hip with the other hand. Ask the patient to bend their hip toward their chest. Grade 2 is assigned if they can extend it with gravity eliminated. If they cannot move it, place the patient in a supine position and palpate the iliopsoas tendon and ask them to flex their hip to distinguish between grade 1 and 0. |
| Knee extension | Extend the knee (in a sitting position). Hold the ankle with one hand to apply resistance, while placing the other under the thigh, proximal to the knee (grade 3, 4 or 5). If grade <3, the patient should lie down on the side not being tested. Support the leg with one hand under the knee and the ankle with the other. Ask the patient to straighten their leg: if they can do this with gravity, grade 2 is assigned. If they cannot move it, place the patient in a supine position and palpate the quadriceps tendon and ask them to push their knee down to distinguish between grade 1 and 0. |
| Ankle dorsiflexion | Patient sits with their heel on the floor (or lying, if they cannot sit up), foot in full dorsiflexion. Apply resistance with one hand on the top of the foot, holding the leg proximal to the ankle with the other (grade 3, 4 or 5). If less than grade 3 but there is partial movement against gravity, assign grade 2. If it is less than grade 2, palpate the tibialis anterior tendon to determine grade 1 or 0. |
MRC: Medical Research Council; ICU: intensive care unit.
An MRC score <48 (average of <4 in the muscle groups evaluated) is considered diagnostic of ICUAW.55,58 The need for the patient to be awake and collaborative is the first limiting factor for its use. Moreover, reported interobserver variability has been variable.59–61 However, an MRC score of <48 is associated with difficulty in weaning from MV, increased length of ICU and hospital, stay and higher costs and mortality.11,12
Manual Prehension DynamometryManual prehension dynamometry has proved to be a useful tool for the study of muscle strength in the ICU. This technique has very good interobserver reproducibility.59,62–64 To carry out the maneuver, the patient must have an MRC ≥3 in elbow flexion and wrist extension. While there are reference values for healthy individuals, values of grip strength (in the dominant hand) <11kg for men and <7kg for women have been used in critical patients to define ICUAW.11,65 Muscle weakness diagnosed by this method has been associated with difficulty in MV weaning, extended ICU stay, and greater in-hospital mortality.11,13
Electrophysiological StudiesElectrophysiological assessment includes the study of nerve conduction, EMG, and the study of the neuromuscular junction.58,66 It can help determine if weakness is caused by muscle or nervous involvement or both. However, the need for equipment and specialized staff, as well as the technical difficulties inherent in critical patients (tissue edema, etc.), means that it is not readily applicable in most ICUs. Recently, the use of simplified electrophysiological studies, such as the peroneal nerve test, showed high sensitivity and specificity in the diagnosis of neuromyopathy in critical patients.67
Peripheral Muscle UltrasonographyUltrasonography has become a valuable tool for the structural assessment of peripheral muscles in the ICU, with an excellent interobserver reproducibility.68–70 Numerous studies have used the thickness or cross-sectional area (CSA) of different muscle groups as a parameter of muscle mass in critically ill patients, demonstrating the progressive development of wasting during the stay in the ICU.54,71–77
Various muscles in both upper and lower limbs have been evaluated. Campbell et al. reported that the combined analysis of muscle thickness in the anterior aspect of the arm, forearm and thigh correlates most closely with lean body mass (in healthy individuals); this method has been subsequently used by several authors in critical patients.73,74,77,78 A correlation between thickness or CSA and muscle strength has been described in healthy individuals and critical patients.75,77,79 The association between muscle wasting and weakness has been demonstrated in septic patients, in whom a further reduction of the CSA of the quadriceps correlated with less strength at the time of ICU discharge.75 However, in a recent study, a single isolated measure of muscle thickness at the time of awakening did not discriminate between patients with and without ICUAW.80 Recently, Puthucheary et al. specifically compared changes in quadriceps thickness with the CSA of the rectus femoris, showing that the latter is a more sensitive and reliable measurement for assessing muscle wasting and weakness in the ICU.81 Moreover, muscle echogenicity increases in critical patients, possibly due to edema, inflammation, fibrosis, fatty infiltration, or necrosis.71,78,82,83 Specifically, an increase in muscle echogenicity predicts the development of myonecrosis (on histology) in the ICU.83 Although an inverse correlation between echogenicity and muscle strength has been established in non-critical patients, this association is not so evident in the ICU setting.71,78
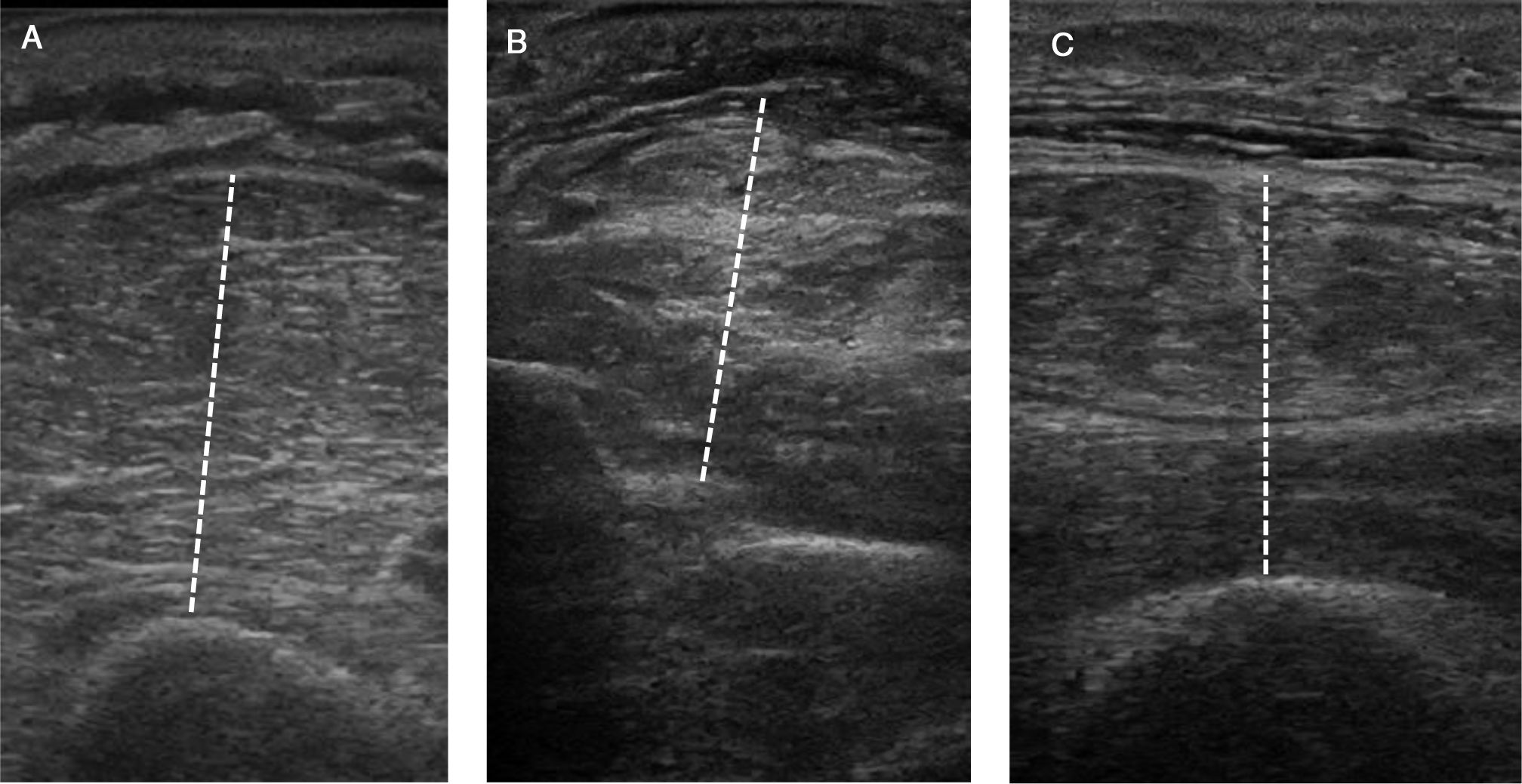
Technique. Several anatomical sites and technical variations have been used in the assessment of peripheral muscle, making it difficult to compare results between studies. Although reference values for muscle thickness and echogenicity have been established in healthy individuals, changes in these values over time seem to be a more valuable parameter in critical patients.84 It is essential that the measurement site and position is precisely identified and standardized. It is important to avoid compression of the muscle during the procedure, so excess conductive gel must be used, and minimal pressure must be applied to the transducer when obtaining the image.
Recordings are obtained with a linear transducer (≥10MHz) positioned in a transverse direction, perpendicular to the underlying bone. The angle of insonation can affect the measurements, so it must be kept constant. Thickness is measured as the maximum distance between the fat–muscle interface and the bone or interosseous membrane (Fig. 2). Table 3 describes the technique for arm, forearm, and thigh.33,73,85,86
Peripheral Muscle Ultrasonography Protocol.
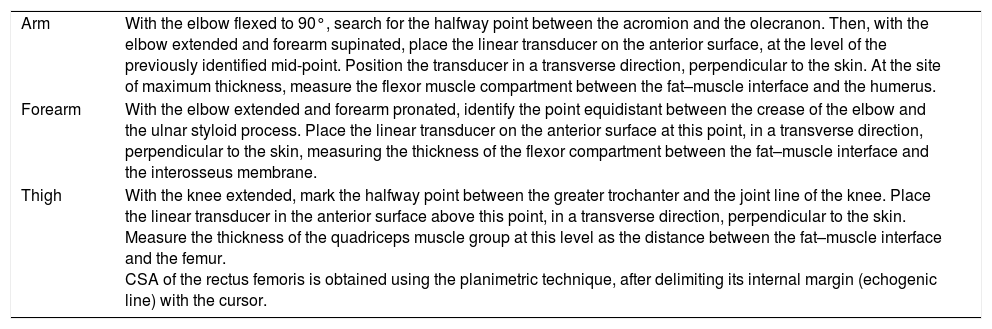
| Arm | With the elbow flexed to 90°, search for the halfway point between the acromion and the olecranon. Then, with the elbow extended and forearm supinated, place the linear transducer on the anterior surface, at the level of the previously identified mid-point. Position the transducer in a transverse direction, perpendicular to the skin. At the site of maximum thickness, measure the flexor muscle compartment between the fat–muscle interface and the humerus. |
| Forearm | With the elbow extended and forearm pronated, identify the point equidistant between the crease of the elbow and the ulnar styloid process. Place the linear transducer on the anterior surface at this point, in a transverse direction, perpendicular to the skin, measuring the thickness of the flexor compartment between the fat–muscle interface and the interosseus membrane. |
| Thigh | With the knee extended, mark the halfway point between the greater trochanter and the joint line of the knee. Place the linear transducer in the anterior surface above this point, in a transverse direction, perpendicular to the skin. Measure the thickness of the quadriceps muscle group at this level as the distance between the fat–muscle interface and the femur. CSA of the rectus femoris is obtained using the planimetric technique, after delimiting its internal margin (echogenic line) with the cursor. |
CSA: cross-sectional area.
Muscle echogenicity can be evaluated semi-quantitatively (Heckmatt scale) or quantitatively using grayscale analysis.70,71,78,82,87
Computed TomographyComputed tomography (CT) is a validated method to determine muscle mass.69 The CSA of muscle tissue in a single slice (usually at the level of the third lumbar vertebra) has been shown to reliably represent body muscle mass.88–90 Moreover, structural muscle changes, such as fat infiltration, can be detected by analyzing muscle density on CT. In critical patients evaluated by CT, muscle wasting, decreased density, and an increase in intermuscular fat are associated with an increase in mortality.90,91 The major limitations of this technique are the need to move the patient and exposure to radiation.
Electrical BioimpedanceElectrical bioimpedance (EBI) analysis can be used at the bedside to determine the patient's lean body mass, although it does not directly quantify muscle mass.92 This can be estimated from regression equations designed for healthy individuals.93 A recent study showed that the capacity of EBI to detect reduced muscle mass in ICU patients is acceptable, although new studies are needed to validate this technique in critical patients.94
BiomarkersThe use of biomarkers could facilitate early diagnosis and follow-up of muscle impairment in the ICU. Various substances have been evaluated for this purpose (myoglobin, creatine kinase, troponin I, etc.). Although high levels have been detected in the context of injury or muscle overload, their usefulness for detecting muscle wasting or weakness in critically ill patients has not yet been demonstrated. Micro-RNAs may represent a new class of biomarkers with enormous potential. It has been suggested that different micro-RNAs participate in the pathogenesis of ICUAW.95–98 Determination of these molecules in plasma might lead to early diagnosis or even predict the development of muscle impairment in critical patients.96 Furthermore, the expression profile of micro-RNAs could vary according to whether the impairment is respiratory, peripheral or both.
ConclusionsMuscle wasting and weakness is a common problem in the ICU, and diagnosis and follow-up can be a real challenge. The use of various clinical parameters and additional methods can facilitate early detection. New non-invasive, accessible, reproducible tools, such as ultrasound, have emerged as valid options for the evaluation of both peripheral and respiratory muscles. The discovery of specific biomarkers will be an area of great interest for future research. The serial evaluation of critically ill patients using ultrasound techniques and the determination of plasma biomarkers could become a new model for the assessment of ICU-acquired muscle wasting and weakness.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Carámbula A, Visca A, D’Amico S, Angulo M. Evaluación muscular respiratoria y periférica en la Unidad de Cuidados Intensivos. Arch Bronconeumol. 2019;55:258–265.