In patients with chronic cough, nearly 40% of the population does not experience definitive improvement of their cough despite correctly applying the anatomic diagnosis. In many of these patients with refractory cough, laryngeal symptoms are frequent. The region of the larynx/pharynx is configured as a bridge between the esophagus and the upper and lower respiratory tract. The association of reflux in patients with chronic cough and symptoms such as globus pharyngis, itchiness or the need to clear one's throat have recently been given attention due to the possibility of joint therapeutic intervention of the gastroesophageal reflux and larynx, both with new medications as well as with laryngeal rehabilitation therapies, with observed benefits in the disappearance of chronic cough in cases that had been previously labeled as refractory.
En los pacientes con tos crónica todavía existe una población cercana al 40% que no mejora definitivamente su tos a pesar de aplicar correctamente el diagnóstico anatómico al uso. En muchos de esos pacientes con tos refractaria los síntomas laríngeos son frecuentes. La zona de la laringe-faringe se configura como el puente entre el esófago y el tracto respiratorio superior e inferior. La asociación de reflujo en pacientes con tos crónica y síntomas como globo laríngeo, picor o la necesidad de aclarar la garganta han merecido atención recientemente por la posibilidad de intervención terapéutica conjunta sobre el reflujo gastroesofágico y la laringe, tanto con nuevas medicaciones como con terapias de rehabilitación laríngea, con beneficios objetivables en la desaparición de la tos crónica en casos que habían sido etiquetados previamente como refractarios.
Chronic cough (CC), defined as cough that lasts more than 8 weeks, is a complex symptom which may be treated by at least 4 different medical specialties: Pulmonology, Allergy and Immunology, Digestive Health and Otorhinolaryngology. Cough is the final result of a reflex mediated by the vagus nerve and it is part of its vegetative function, along with swallowing and voice. The clinical diagnosis of CC is based on either a cause–effect relationship (asthma, gastroesophageal reflux [GER] and upper airway resistance syndrome) or on diseases that do not necessarily involve cause–effect (COPD, cancer, heart failure).1 In this review, we will exclusively discuss the former group.
Once eosinophilic inflammation of the respiratory tract has been ruled out (asthmatic bronchitis and non-asthmatic eosinophilic bronchitis), in cases of cause–effect CC there is more and more evidence to consider sensorial disorders of the of the vagus nerve as the most important etiology, giving rise to the so-called chronic laryngeal cough (CLC). We use the term CLC to define cough that originates in a disorder of the vagus branch that innervates the larynx, where there are more cough receptors than in other places. In these cases, it is very possible to detect the coexistence of laryngopharyngeal reflux (LPR), which is the reflux that ascends to the larynx from the esophagus. In addition, since the vagus innervates the entire aerodigestive tract, there is growing evidence of the simultaneous stimulation of different afferent branches that constitute the reflex arch of cough, in this case from the larynx and esophagus. A global vision of both laryngeal and esophageal disorders in patients with chronic cough is becoming more necessary, and we present the basis for this new orientation in this document.
Patients with CC who are treated in otorhinolaryngology, unlike patients with CC originating in the lungs, almost always demonstrate cough-related symptoms such as pharyngeal clearing or hoarseness, throat itchiness, globus pharyngis, dysphagia, dysphonia, dyspnea and/or stridor. However, even the diagnosis of CC associated with eosinophilic inflammation of the airway, a finding that is a priori specifically pulmonary, can be related to GER.2 The clinical guidelines for CC rarely consider CLC3,4 because either the symptoms of the cough of this origin are confused with cough caused by extraesophageal reflux5 or because the CC may not be the predominant symptom, as it usually occurs in CLC6 and as we have previously mentioned. This lack of agreement in the consideration of CC of diverse origins (due to the lack of consensus between otolaryngologists, gastroenterologists and pulmonologists) is most likely responsible for the high frequency of unexplainable or idiopathic CC, which is consequently often refractory to standard treatment.
The prevalence of unexplained CC is variable, but there are published reports of it reaching up to 42% of cases.7 This has led some of the researchers to suggest that a new focus is needed to manage chronic cough, especially CC originating in the upper airway. The larynx may play an essential role in cases of refractory CC. This argument is justified by the fact that it is the origin of an important flow of afferent impulses of the cough reflex due to its anatomical position as a link or bridge between the esophagus and the tracheobronchial tree. Furthermore, the coexistence in the larynx of cough and the laryngeal closure reflex is not only important to protect the airway during swallowing, but it is also the point of origin of cough as it is the first station for potentially harmful inhaled stimulants. The sensory receptors that incite the closure of the vocal chords and cause cough as part of the glottic closure reflex are found not only in the larynx but also in the trachea and the upper respiratory tract. They respond to pressure as well as to irritating stimuli. It is therefore not strange that patients with CLC have an added sensation of respiratory difficulty.
It is currently considered that, in general, the patient population with reactive airway disease is grouped into 2 types. One is the group of patients with asthma, meaning those with reactive disease of the lower respiratory tract. The other smaller group consists of patients with reactive disease of the upper airways, who develop obstruction in the larynx, meaning with a variable degree of vocal cord adduction. The hallmark of the former group is wheezing, and of the latter it is stridor. Many patients with laryngospasm or paradoxical vocal cord motion (PVCM) are incorrectly diagnosed with asthma, although with careful analysis of the patient files it is almost always possible to discover that these patients have inspiratory stridor and not wheezing.8
The 3 terms that are most frequently found in the literature for disorders whose predominating symptom is cough originating in the larynx are: PVCM, vocal cord dysfunction (VCD) and laryngospasm. All three have in common either permanent or intermittent closure of the glottic area. PVCM is usually the denomination preferred by otolaryngologists. Contrarily, VCD is more often used by pulmonologists and allergists. Do these terms represent exactly the same disorder with a common origin? Many recent studies not only identify the population at risk, but also the common symptoms that they present, among these CC, possible physiopathology for vagal neuropathy and several very promising treatments.9–13
Protocols for the diagnosis and treatment of CC have generally been developed by pulmonologists,3,4 but now otolaryngologists have shown new perspectives in its treatment and management11 by arguing that a sensory neuropathy of the larynx may be responsible for many cases of refractory CC.
Mechanisms of Chronic CoughCough and breathing disorders have in common afferent sensory stimuli that originate in the aerodigestive tract and are directed toward the brainstem.14 The cough reflex is triggered by a stimulus in the upper aerodigestive tract that excites the sensory receptors, which in turn send afferent information mediated by sensory neuropeptides. The integration of this information results in motor commands from the brain and brainstem that make up the efferent pathway of cough. Today we know that one cough stimulus alone cannot provoke the cough reflex, and that it is supposedly necessary for there to be integration among different afferent branches of the vagus nerve to bring about cough.15 This multifactor concept of the origin of CC represents a new perspective, not only for its diagnosis but also for treatment.
The afferent branch of the vagus nerve innervates extensive areas of the aerodigestive tracts, and it is responsible for the origin of the cough reflex. These areas include the ears, pharynx, larynx, lungs and the areas innervated by gastric, cardiac, esophageal and intestinal branches of the vagus. There are 7 types of intraepithelial sensory receptors involved in the cough reflex. The most important are the rapidly adapting receptors (RAR) and the bronchial C-fiber receptors. Both also conciliate bronchial constriction stimuli, including chemical and mechanical agents, as well as other inflammatory mediators.16 In general, the distribution of the receptors is mostly of the mechanosensitive type in the area of the larynx, and more of the chemosensitive type in the distal airway.14
The sensitivity of the cough reflex in humans is linked to 2 types of afferent pathways.17 One is induced by mechanosensory stimuli that are transmitted through myelinated nerve fibers, which are not chemically reactive (meaning that they do not respond to the capsaicin test) and whose primary function is to prevent aspiration in the lungs. The other is the chemosensory pathway that is made up of group C nerve fibers without myelin, and the transmission mechanism is analogous to that of pain induced by tissue injury and is controlled by transient receptor potential cation channels, especially TRPV1 and TRPA1 or “irritant sensor”. Cough appears as the only response of the lower respiratory tract to the inflammatory lesion as there is no response to pain. Both sensory afferent pathways end at the brainstem, at the nucleus of the solitary tract. The cough center may be influenced by the voluntary movement area of the cortex, since the cough reflex can decrease when the state of consciousness is depressed. The cough center is probably different from the medullary respiratory center, and in animal models it has been demonstrated that, although general anesthesia can depress the respiratory center, the cough reflex remains intact.
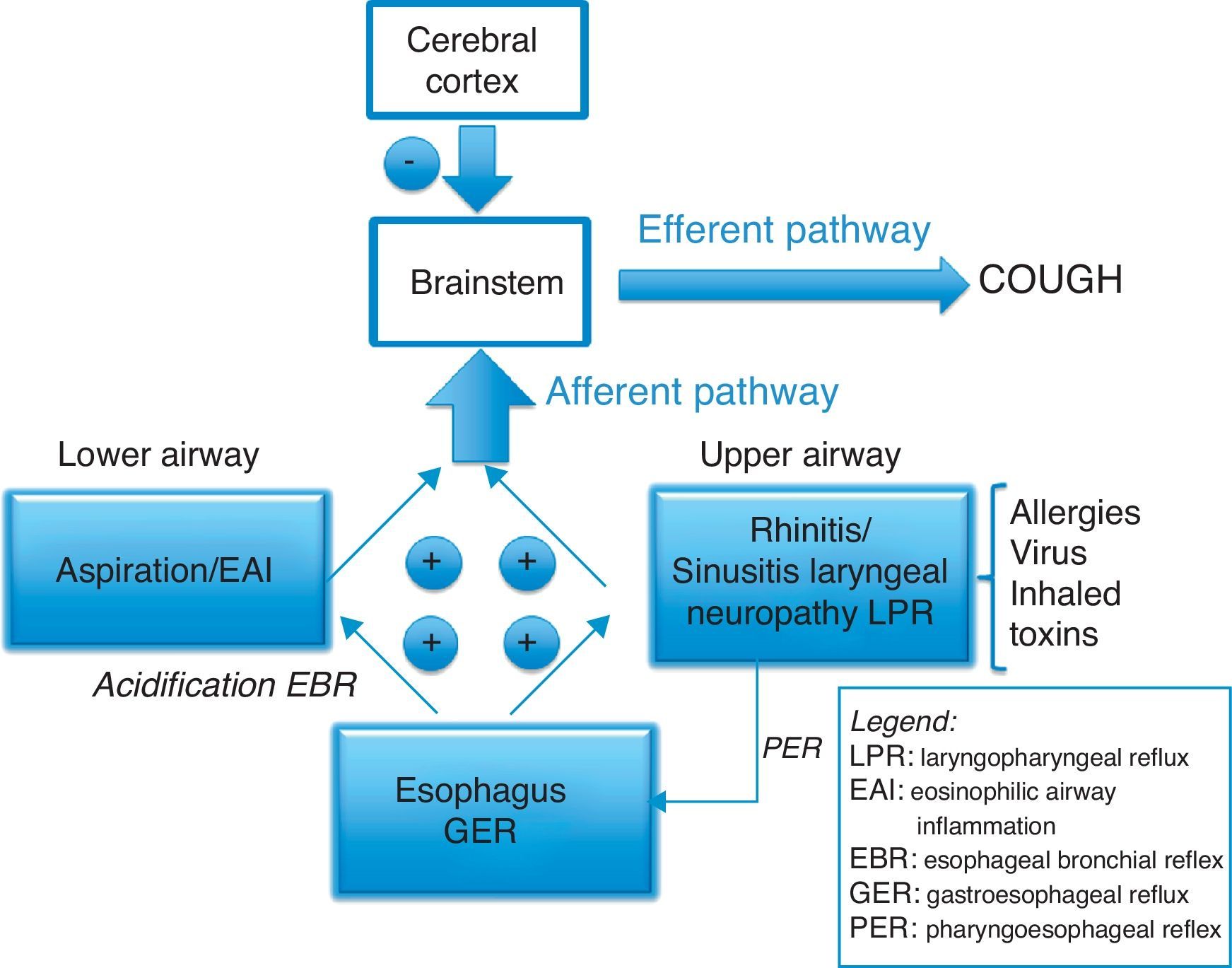
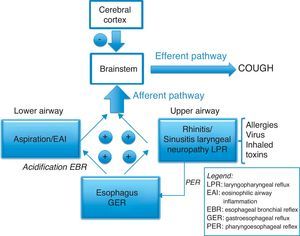
A general, widely accepted view is that the chronic increase in the sensitivity of the cough reflex arc is associated with eosinophilic inflammation of the respiratory tract, gastroesophageal reflux or upper airway alterations, after having ruled out the use of ECA inhibitors, exposure to tobacco smoke, COPD or an evident pathology on thoracic CT.3 But the relationship at the neuronal level between the esophagus, larynx and lungs is complex. Two reflexes have recently been described, one being the esophageal–bronchial reflex induced by the distal acidification of the esophagus18 and the other being the laryngoesophageal reflex that can cause, from the larynx, the opening of the lower esophageal sphincter and cause reflux19 (Fig. 1). Furthermore, it has been recently proven by Australian specialists that the triggers that stimulate the afferent pathway of cough constitute an important part of the reflex arc,12 since low-intensity stimuli such as speaking, laughing, scents, stress, smoke, etc., can spur the cough reflex. For this reason, more studies about this topic are accepting that CC is a neuropathic sensorial disorder.
Before the onset of CC, most of the patients deny having any typical symptoms associated with CLC such as laryngeal clearing or clearing of the throat, dysphonia, a sensation of globus pharyngis or itchiness. In the initial stages of CC, this lack of typical symptoms poses at least 2 problems in its interpretation. First of all, there are several areas that can stimulate the cough reflex at the same time, a fact that has been confirmed in 62% of patients studied by Irwin et al.3 From our own experience, in an analysis of 270 patients with CC, we have confirmed that 84% of cases had 2 or more potential sites where the cough had originated.20 Secondly, it is frequent for the medical files of individuals with CC to include the presence of cough triggers or laryngeal paresthesia, especially in refractory CC.12 Furthermore, if we analyze reports of patients with CC treated with the universally accepted anatomic diagnostic protocol, rarely do we see the complete disappearance of the cough mentioned. There are merely descriptions of improvements of different magnitudes. We should therefore accept that treatments aimed at a single specific cause of CC, as is the current norm, are often not totally effective.
CC originating in the laryngeal area (CLC) has merited growing interest lately and has been associated with states of hypersensitivity or hyposensitivity of the laryngeal mucosa. Morrison et al.21 presented for the first time the concept of irritable larynx, which has been profiled over the years and has received several names: postviral vagal neuropathy, sensory neuropathic cough and laryngeal sensory neuropathy. There is great confusion created by the terminology used, and it is probably due to this fact that references made about this pathology have had little impact. Vagal neuropathy can affect the motor branches of the vagus nerve, translating into paralysis or paresis of the vocal cords, and can also affect the sensorial branches. This can lead to not only CC but also laryngeal paresthesia, including sensations like itchiness, globus pharyngis, excessive pharyngeal mucus, odynophonia or laryngeal spasms, with the particularity that they occur without exposure to triggers.22 These symptoms can become aggravated by localized efforts such as prolonged phonation, and by irritating stimuli, which we can often confirm when triggering discomfort by lightly palpating the cricoid cartilage.
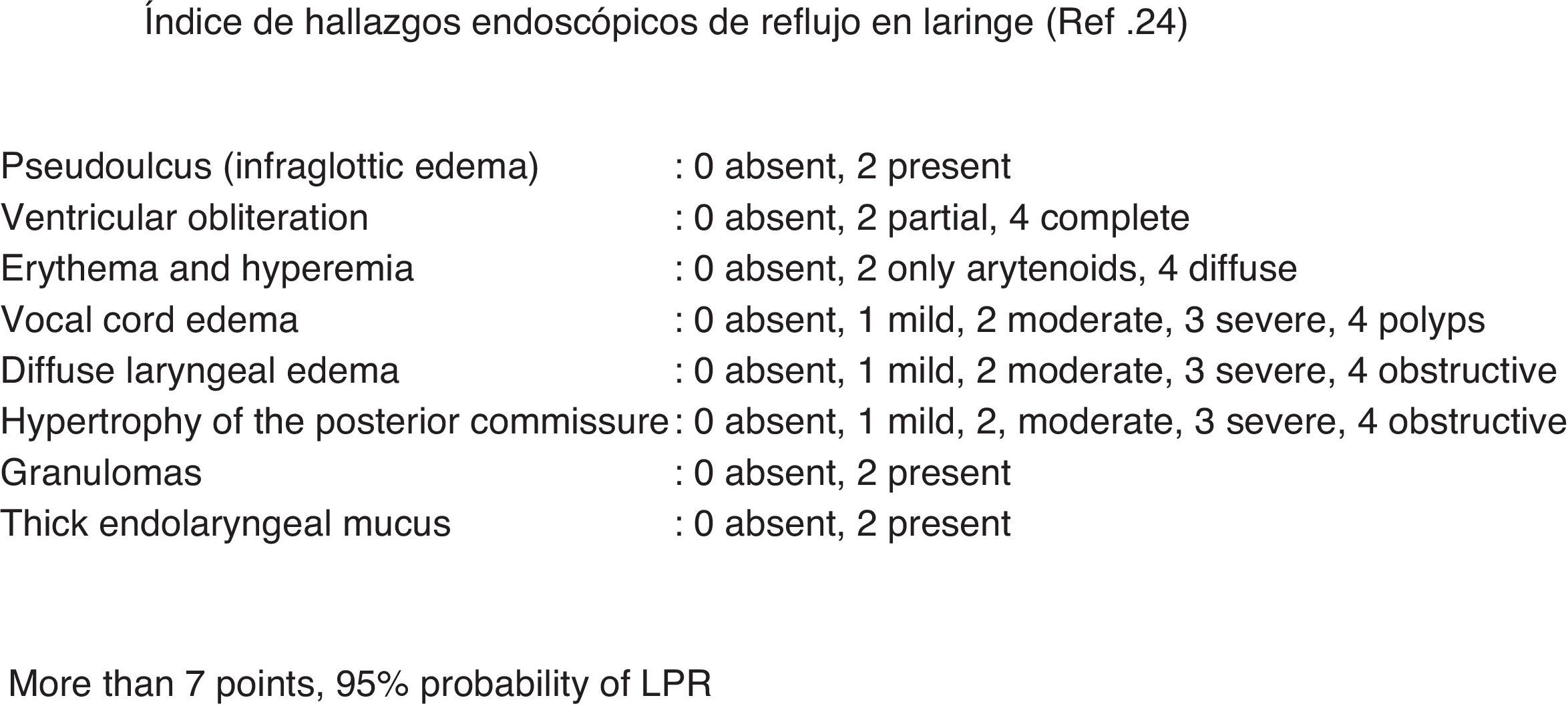
Authors like Morrison et al. suggest that LPR is the agent that magnifies the symptoms in more than 90% of patients with acute or recurrent irritable larynx syndrome.21 Moreover, there are many studies that have demonstrated that the irritation caused by LPR is the cause of the lack of laryngopharyngeal sensitivity in patients with CC and GER compared with control subjects.10,23,24 In our group, we developed a study that was presented at the SEPAR Congress in 201225 that included 170 patients with CC and GER. We observed that 34% presented a positive endoscopic reflux index, which is a universally accepted objective test for LPR26 (Fig. 2). Rees et al. have also described that one-third of patients with CC and postviral vagal neuropathy had LPR.13 At the same time, Murry et al., in an interesting paper about the relationship between CC and PVCM, observed a reduced sensitivity to mechanical stimuli in the laryngeal mucosa in patients with LPR.10 In addition, in those larynxes with reduced mechanosensitivity, paradoxically, a state of sensory irritant alteration can occur, which may cause irritating cough as a main symptom, along with other symptoms like itchy throat, sensation of globus pharyngis or foreign body, excessive pharyngeal mucus and even laryngospasm. It is therefore reasonable to theorize about the existence of an anatomical and physiopathological connection between CLC and LPR.
Some authors like Morice support the idea that CC actually is part of a more extensive syndrome, known as Cough Hypersensitivity Syndrome (CHS). The physiological base of CHS is reflux, both liquid as well as gaseous, acid or non-acid, and includes several phenotypes such as eosinophilic airway inflammation, GER or affectation of the upper airway. Symptoms relating with the larynx are very frequent in this syndrome, and the author justifies his more global hypothesis for CC because: (i) in the larynx, there is a greater density of cough receptors; and (ii) there is evidence of a common clinical history among all the chronic cough phenotypes when the Hull Cough Hypersensitivity Questionnaire is applied.27
Chronic Cough Associated With Laryngeal Neuropathy and Laryngopharyngeal RefluxAn important characteristic of neuralgia is the presence of the trigger phenomenon or reduced provocation threshold. It has been speculated that neuralgia of the upper laryngeal nerve could be noted in the larynx as a sudden and exaggerated sensation, although not painful, that would lead to an uncontrollable cough or urge to cough. The idea is that the threshold for the initiation of the cough reflex decreases significantly. Therefore, in this situation, with minimal stimuli and even with no stimuli at all, the need to cough may arise. Lee and Woo suggested that untreatable CC could also be a manifestation of a laryngeal sensory neuropathy observed with electromyography of the cricoid muscles and/or stroboscope.28 Amin and Koufman9 described for the first time the association between cough due to laryngeal neuropathy and prior viral infection of the respiratory tract.
But the interesting idea is that CC can be a synergic, integrated response to laryngeal stimuli and irritating stimuli of the reflux components, although several erroneous hypotheses are maintained and keep this association from being completely accepted. CC is a common symptom in medical practice and its relationship with reflux still has not been completely proven, despite the fact that a characteristic clinical history can be identified together with proven gastroesophageal disease in patients with CC.5 In patients with CC, if allergy tests, CT of facial sinuses and thorax, as well as lung function tests are all normal, it is almost doubtless that the cause of the cough is GER.29 In fact, Irwin indicated that CC may be the only symptom in patients with GER. In addition, it has also been recently demonstrated that in patients with CC, proximal non-acid reflux is most important19; therefore, it is no wonder that GER can be clinically silent (i.e. without pyrosis) in up to 75% of patients with CC who are studied.
One of the problems with assigning specific causes to CC has to do with the objective diagnosis of GER. The functional diagnosis of GER can be verified in 3 ways: maintained exposure of acid in the distal esophagus (pH meter), microaspiration due to the inefficacy of the upper laryngeal sphincter (double pH meter study) or ineffective esophageal motility that leads to prolonged acid clearance time.30 LPR is often considered by otolaryngologists as a frequent cause of CC, but pulmonologists still need to confer LPR greater recognition because it is argued that there is some overlapping between the phenotype of patients diagnosed with cough due to GER and cough due to LPR with patients with CC due to laryngeal neuropathy.31
The physiopathology of CC associated with GER has 2 mechanisms: (1) exposure to acid in the distal esophagus that triggers the esophageal bronchial reflex and (2) microaspiration of esophageal content, which ascends to the larynx and later penetrates the tracheobronchial tree. This aspiration mechanism is called indirect in order to differentiate it from direct aspiration, which is when the pharyngeal bolus enters the trachea directly without passing through the esophagus (Fig. 3). The first mechanism takes place in distal GER and the latter is due to proximal GER or LPR. Isolated monitoring of esophageal pH does not detect all the refluxes, particularly those with little or no acid content, but nevertheless may be of respiratory interest. In addition to acidity, the gas–liquid composition of the reflux can also be relevant in the pathogenesis of LPR. Two recent studies, for example, have shown that regurgitation and cough, not pyrosis, are symptoms that are most frequently associated with non-acid reflux.32,33 In addition, although the diagnosis of LPR can be done acceptably with a double-junction pH meter, Wo suggests34 that there is no convincing evidence that monitoring proximal esophageal pH can predict the response to treatment with IBP in patients with symptoms of LPR, since the technique can only measure liquid acid reflux. This has led to more detailed monitoring of the laryngopharyngeal area. Moreover, the original methods for measuring pharyngeal pH were not all that correct due to technical problems such as catheter desiccation or the accumulation of mucus and the presence of food remains. The Dx-pH monitoring system (Dx-pH; Restech Corporation, San Diego, CA) is a new device that is very sensitive and minimally invasive used to detect acid reflux at the posterior pharyngeal wall. This sensor detects acid both in aerosol as well as liquid states, resists desiccation and its signal transmission is not affected by contact with liquids or organic matter. Wiener compared a traditional 24-h esophageal pH meter with the Dx-pH in 15 patients with extra-esophageal symptoms. All the events measured with the Dx-pH method were progressively preceded by drops in distal esophageal pH.35 In addition, some studies about oropharyngeal pH with the Dx-pH catheter showed a growing pH gradient from the distal esophagus to the oropharynx. The oropharynx, in general, presents a slightly acid pH that is rarely below 4. This could explain why previous studies have not been reliable when aiming to distinguish normal subjects from the group of patients with symptoms of extra-esophageal GER or atypical symptoms when the pH cut-point <4 was immovable.
Blondeau et al. have developed a statistical index for the temporal association of reflux and cough, known as symptom-associated probability (SAP). In their study, a positive result for SAP was only found in a small proportion (5%) of the patients with chronic cough and cough caused by episodes of GER.36 Nevertheless, in accordance with the recent research by Canning, it is necessary for there to be signals from several origins in order to trigger the cough reflex.15 Thus, in patients with cough due to GER, in addition to sensitization to reflux in the esophagus, it is possible for other cough stimuli to simultaneously coexist, and it is therefore necessary to extend the study by, for example, simultaneously analyzing inflammation in the lower respiratory tract2,18,37 or inflammation of the upper respiratory tract.38 This research could be the subject of future studies.
After a viral infection of the upper airways that runs its course with frequent cough, the patient usually denies previously having any typical LPR symptoms. Therefore, the simultaneous appearance of LPR and CC after an infection of the upper respiratory tract suggests the possibility that the viral inflammation may have also affected the branches of the vagus that innervate the esophagus and its sphincters. In the larynx, a state of acute hypersensitivity is probably secondary to the lesion of the vagus nerve. But, when chronic hypersensitivity develops, for example, due to inflammation from recurrent reflux episodes, the consequence could be an anomalous generation of the nerve or an alteration in the cough center of the CNS. The idea that patients with CC originating in the larynx, in whom there is a predominance of laryngeal paresthesia mainly caused by a state of chronic hypersensitivity, could justify the recently published success in the readaptation or rehabilitation of the respiratory tract in patients with refractory CC.10–12 There are also studies that demonstrate that cough associated with PVCM can be attributed to a decrease in the sensitivity of the receptors located in edematous laryngeal mucosa. It is interesting to confirm that the reduced mechanosensitivity and the increased chemosensitivity of the laryngeal mucosa due to the acid irritation from the digestive tube could cause an accumulation of particles or irritants in the area of the larynx. Therefore, the chronic cough reflex could simply be an adaptive mechanism for clearing the larynx.10 Cukier-Blaj argued that the irritation of the larynx caused by reflux reduces laryngeal sensitivity, which leads to a compensatory motor response with hyperadduction of the vocal folds during inspiration, with cough and dysphonia.39 Another theory proposes that chronic irritation of the larynx due to GER, with evident symptoms of laryngeal clearance, predisposes the larynx to be more sensitive to different external stimuli, creating a vicious circle.21 According to this hypothesis, after the initial exposure many patients state having developed “hypersensitivity” over time to several triggering factors like tobacco, cold air, exercise, perfume, soap, other fragrances and emotional stress. This clinical presentation often leads to incorrect diagnoses like asthma or multiple chemical sensitivity. The chronicity of the symptoms in this pathology means that patients are frequently and unsuccessful treated for asthma or other comorbidities before the correct diagnosis is made. There are patients with concomitant asthma and LPR, but there are others where CC is associated with other symptoms produced by the LPR such as episodic sensation of asphyxia and lack of air, starting with respiratory symptoms at the same time. In these cases, LPR seems to be either the cause of, or at least a decisive factor in, the respiratory problems. In conclusion, more studies are needed to definitively clarify the causal relationship of the variety of symptoms and stimuli of different origins that may present simultaneously in a patient with CC.
Chronic Cough as Hypersensitivity SyndromeChronic cough hypersensitivity syndrome (CCHS) has been proposed to identify a group of patients with unexplained chronic cough (UCC), for whom no morphological reason has been found to justify their CC by using conventional anatomic diagnosis, but who maintain a notably increased sensitivity to capsaicin. This suggests abnormality in the sensory nerves of the respiratory tract. The sensory receptors that mediate cough are not completely known, but the transient receptor potential (TRP) channels, from the family of ion channels, are strong candidates and it has been demonstrated that there is a greater expression of TRP-1 vaniloid in the nerves of the respiratory tract of patients with UCC.40 This finding reinforces the concept of CCHS with an unquestionable physiopathological base.
The symptoms that are generally associated with CCHS include a tingling or persistent irritating sensation that the patient locates in the chest or throat, scratchy voice, dysphonia or PVCM.1 A high sensitivity to the cough reflex may be considered reversible or persistent in patients with CCHS, and the cough is generally dry or minimally productive. Nevertheless, the hypersensitivity of the respiratory tract nerves cannot be limited to only those related with cough. Thus, the increased sensitivity of the laryngeal closure reflex and the high prevalence of vocal cord dysfunction and voice disorders in CLC increase the possibility that CC is actually a generalized alteration in the innervation of the respiratory tract.6,38 In addition, if strange sensations or laryngeal/tracheal paresthesia and the activation of the cough reflex arc after low-level stimuli or even without stimuli are the characteristic findings of a sensory neuropathic alteration as mentioned above, the positive response to recent neuromodulator therapies like gabapentin or amitriptyline, has reinforced the consideration of CC as a sensory neuropathy41–44 originating where there are the most cough receptors, which is the larynx – thus the term CLC. Other CC studies such as that by Morice, argue that CCHS is a more universal concept for CC because it would span all its phenotypes, including CLC, with the common etiologic factor of GER.
Recent research describes the importance of refractory CC originating in the upper airway. Up to two-thirds of patients diagnosed with CC are able to identify the stimuli that cause their cough45; however, laryngeal paresthesia, as part of the symptoms profile of CC, was present in 94% of the patients with CC that was refractory to medical treatment and whose diagnosis had been based on the anatomic diagnosis protocol.11 We therefore believe that if we group the sensory alterations of the laryngeal mucosa with the denomination of CLC under the heading of CCHS, the anatomic-diagnostic guidelines of CC come full circle, and CC is established as a global concept of anomalous vagal reflex arc with different sources of origin.
Treatment of Chronic Cough Related With Vagal NeuropathyMost of the patients with CC have rhinosinusitis, eosinophilic inflammation of the airways, allergies or reflux and have varying responses to the specific therapies for these pathologies. However, those who do not respond to empirical treatment should undergo a series of tests to try to diagnose CLC.12 In a recent study carried out by pulmonologists that entailed a long-term follow-up in patients with inexplicable CC, it was observed that the cough remained in most of the patients, although the intensity decreased in more than 50%.31 Logopedic therapy could be a useful treatment for refractory CC, providing prolonged results. Ryan demonstrated that voice rehabilitation is an effective treatment for refractory CC and that the mechanism that it is based on is due to the reduced sensitivity of the cough reflex arc, showing reduced urge to cough and increased cough threshold.46 Another study by Vertigan et al. demonstrated that CC that persists despite medical treatment may respond to logopedic intervention with a success rate of 88% vs 14% in the placebo group.11
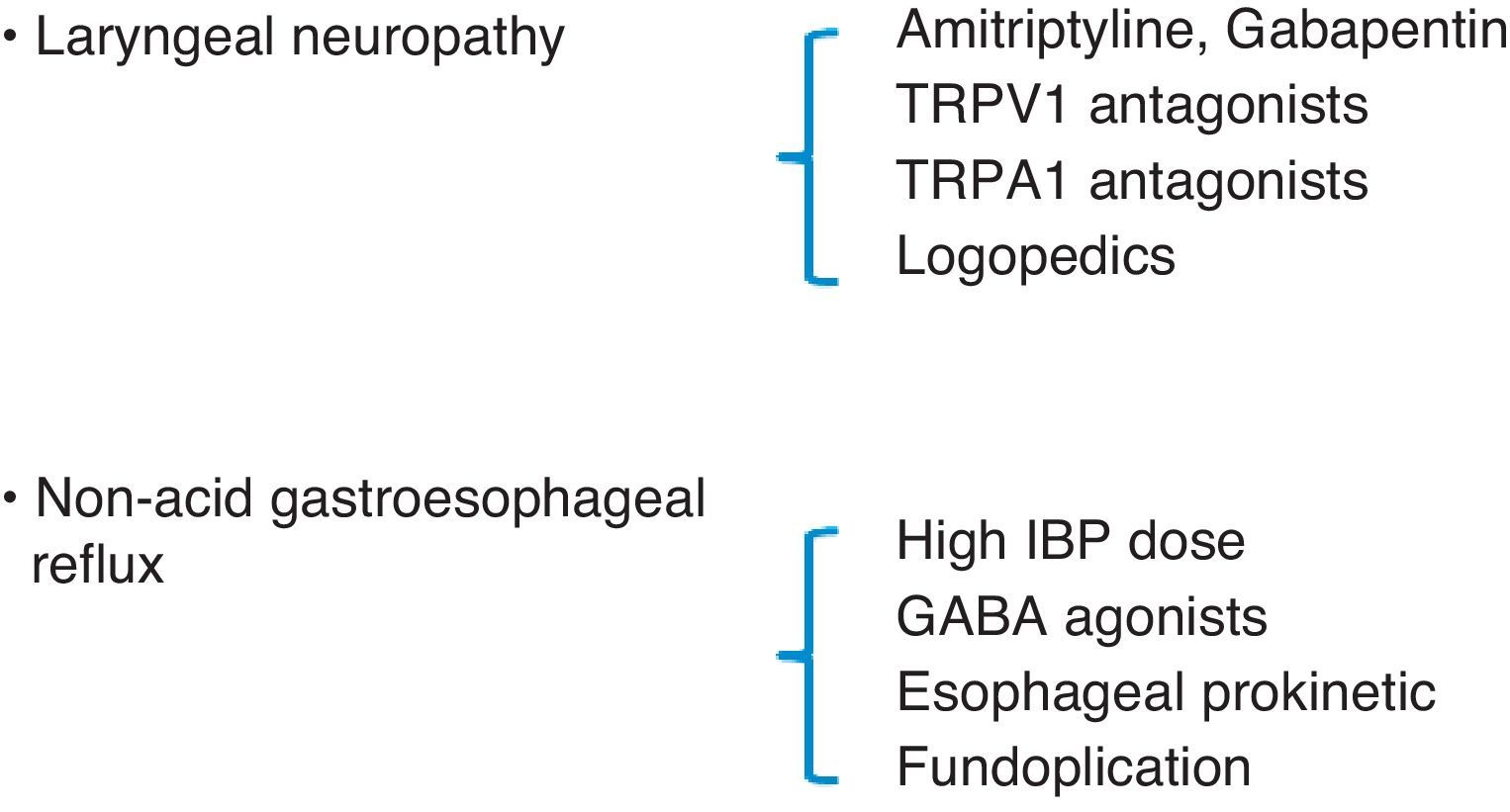
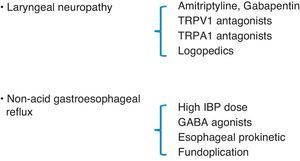
Lee believes that CC may be a sign of laryngeal sensory neuropathy and, therefore, as occurs with neuropathic pain, it may improve with neuroleptic drugs.28 In open studies with drugs that have an effect on the peripheral nerves and are used to treat neuropathic pain, like amitriptyline and gabapentin, these medications have been found to reduce cough severity.43,44 Currently, it is not clear what dosage of amitriptyline could interfere in the cough reflex. Nevertheless, recent publications about the neurologic mechanism of pain suggest that amitriptyline has a quick, long-lasting effect on the nerve that is better than a classic local anesthetic. Its effectiveness could be due to its ability to block cholinergic/muscarinic receptors or histamine H1 receptors instead of blocking neuropathic pain transmission.47 In the case of a synchronic diagnosis of LPR in a patient with CC, maximal anti-acid therapy should be added by doubling the dose of IBP (40mg/12h) and adding a nighttime dose of H2 inhibitors (ranitidine 300mg) to impede excess nighttime acid secretions,48 and this treatment should be prolonged until the symptoms, especially CC, resolve themselves or are controlled, after which time the treatment should be continued for an additional period of 3 months.49 If non-acid reflux is observed, GABA agonists (baclofen) have been demonstrated to be effective due to their inhibitory function of the number of transitory relaxations of the lower esophageal sphincter.50 Fundoplication, as a last resort in cases of non-acid refluxes with no response to medical treatment, may be appropriate in some patients with CC.51,52
In conclusion, the new findings about the cough reflex and the interpretation of the symptoms that accompany it support the hypothesis that CC can be multifactorial in origin and that, within its diversity, the larynx as the location for chronic affectation of the vagus nerve now constitutes a new diagnostic perspective. Recent advances in the management of these patients include the introduction of logopedics therapy and treatment with neuromodulator drugs, especially in those individuals whose cough persists in spite of standard medication. Furthermore, taking into account the fact that CLC and LPR frequently coincide, double therapy directed at both entities is recommended from the start. All these treatment objectives mean that, in any given patient, there may be the need for a coordinated effort between pulmonologists, otolaryngologists, gastroenterologists and speech therapists in order to obtain total success in the treatment of chronic cough.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Pacheco A, et al. Tos crónica refractaria. Nuevas perspectivas en diagnóstico y tratamiento. Arch Bronconeumol. 2013;49:151–7.