Lung cancer (LC) is a major public health issue. Despite recent advances in treatment, primary prevention and early diagnosis are key to reducing the incidence and mortality of this disease. A recent clinical trial demonstrated the efficacy of selective screening by low-dose computed tomography (LDCT) in reducing the risk of both lung cancer mortality and all-cause mortality in high-risk individuals.
This article contains the reflections of an expert group on the use of LDCT for early diagnosis of LC in high-risk individuals, and how to evaluate its implementation in Spain. The expert group was set up by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), the Spanish Society of Thoracic Surgery (SECT), the Spanish Society of Radiology (SERAM) and the Spanish Society of Medical Oncology (SEOM).
El cáncer de pulmón (CP) constituye un problema de salud pública de primer orden. A pesar de los recientes avances en su tratamiento, la prevención primaria y el diagnóstico precoz son las claves para reducir su incidencia y mortalidad. Un ensayo clínico reciente demostró la eficacia del cribado selectivo con tomografía computarizada de baja dosis (TCBD) en la reducción del riesgo de muerte en personas de alto riesgo, tanto por CP como global.
Este artículo recoge las reflexiones de un grupo de expertos designados por la Sociedad Española de Neumología y Cirugía Torácica (SEPAR), la Sociedad Española de Cirugía Torácica (SECT), la Sociedad Española de Radiología Médica (SERAM) y la Sociedad Española de Oncología Médica (SEOM) sobre el uso de la TCBD para el diagnóstico precoz del CP en personas con riesgo elevado de padecerlo y los pasos necesarios para evaluar su implementación en nuestro país.
Lung cancer (LC) has gone from being a rare disease at the beginning of the 20th century to being the major cause of cancer mortality in industrialized countries.1,2 In 2012, over 1.8 million cases were diagnosed worldwide and 1.5 million patients died of this disease. In Spain in 2013, 21664 patients died of LC (17559 men and 4105 women),3 accounting for 19.5% of all cancer deaths. The incidence of this disease is no longer rising so fast, having fallen from 29.4% in the 5-year period from 1980 to 1985 to 5.1% from 1995 to 2000, but the number of new cases continues to grow: in 2013, LC increased by 0.8% in men and 7.3% in women compared to the previous year.4
Despite advances in the diagnosis and treatment of LC,5 5-year survival for all stages in Europe ranges from 9.6% in the United Kingdom to 17.9% in Austria, while in Spain, 5-year survival, at 12.6%, is lower than in the United States (18.7%), and particularly in Japan (30.1%). In Spain, incidence and mortality due to lung cancer in men are close to the European average, with a trend toward stabilization. However, although the incidence of LC in women is among the lowest in Europe, it is clearly on the rise.6,7 The current men to women ratio of LC incidence is 4.2:1.0.3 Median age at diagnosis of LC in Spain is 69 years for women and 70 years for men.8
LC survival is associated with disease stage at time of diagnosis. Unfortunately, most LCs are still diagnosed at advanced stages, explaining why the 5-year survival rate for all patients is less than 15%.9,10
Strategies aimed at reducing tobacco consumption have had the greatest impact on LC mortality.11 During the last few decades, the value of different radiological techniques and biological markers (e.g., sputum cytology or serum biomarkers) has been investigated, but positive results were only achieved following the publication of studies on the usefulness of screening with low-dose computed tomography (LDCT). Following in the footsteps of various medical societies that have made their positions public,12 this article contains the reflections of a group of experts designated by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), the Spanish Society of Thoracic Surgery (SECT), the Spanish Society of Medical Radiology (SERAM) and the Spanish Society of Medical Oncology (SEOM) on the use of the LDCT for the early diagnosis of CP in high-risk individuals, as well as the steps needed to evaluate the implementation of this procedure in our country.
Evidence on the Use of Low-dose Computed TomographyOne of the first studies to analyze the utility of LDCT to improve LC diagnosis was the National Lung Screening Trial (NLST),13 which included 53454 smokers and ex-smokers aged 55–74 years with a minimum consumption of 30 pack-years, or former smokers with less than 15 years of abstinence. Screening was performed annually over a 3-year period, and the usefulness of LDCT was compared with that of standard chest radiography. Results showed a relative reduction of 20% in death due to LC in the LDCT group compared to the chest radiography group (95% confidence interval [CI]: 6.8%–26.7%; P=.004), and a 6.7% reduction in overall mortality (CI 95%: 1.2%–13.6%; P=.02). The rate of major complications associated with LDCT was 0.06% in positive cases that finally did not have LC, and 11.2% in those who did, and the surgical mortality rate was 1%. The study was stopped before completing the planned follow-up, after the minimum established endpoint of reduction in mortality was reached.
The NLST study was preceded by 3 randomized trials which found no reduction in mortality compared to the control group.14–16 The investigators of another comparative European study with a larger sample size (Nederlands Leuvens Longkanker Screenings Onderzoek [NELSON]) have published data on the characteristics of the tumors observed in their study, but the mortality results are still pending.17
The International Early Lung Cancer Action Program (I-ELCAP) prospectively recorded survival in patients with stage I LC diagnosed by LDCT.18 It enrolled 31567 non-randomized asymptomatic volunteers at risk of developing LC; 484 developed LC and 85% were diagnosed in stage I, with an estimated 10-year survival of 88%. The survival rate of the 302 patients with stage I LC who underwent surgical resection within 1 month of diagnosis was 92%.
Limitations of the NLST StudyDespite the NLST outcomes, generalized use of LDCT has been limited by some concerns, such as low specificity, overdiagnosis, and fear of radiation. In the NLST follow-up, 112 more cancers were diagnosed in the LDCT group than in the chest X-ray group. These data suggest a rate of overdiagnosis of between 11% and 18%.13 The Danish Lung Cancer Screening Trial (DLCST) also found more tumors in the LDCT screening group than in the control group.14 However, a pathology study of the I-ELCAP cohort confirmed that 95% of the tumors diagnosed by LDCT showed signs of invasion, so the rate of overdiagnosis may be lower.18
Specificity of the LDCT findings is limited by the finding of benign nodules. In the NLST study, if a cut-off point of a diameter of 4mm was selected, 96% were false positives (FP). Although most positive results in the NLST study led to follow-up with LDCT alone, 1.8%, 3.8% and 4% of subjects with a positive result in any of the three rounds of screening were subjected to percutaneous aspiration, bronchoscopy or surgery, producing at least 1 complication in 1.4% of the LDCT screening group, and 1.6% in the X-ray screening group. The complication was deemed relevant in 0.06% of cases. Moreover, 0.9% of all positive subjects were subjected to surgery in which no LC was detected.13 The NELSON study showed that FPs are reduced by analysis of volume-doubling time in nodules detected on LDCT (2.6% in the baseline study and 1.8% in the subsequent annual control), with no increase in false negatives (FN).19 The NLST study included an analysis of stress associated with screening results in a subgroup of patients with a positive result, which found that neither quality of life nor the degree of anxiety were affected by such a result.20 In contrast, in the NELSON study, an analysis of the short-term effects on quality of life showed that in a subgroup of individuals with an indeterminate result in the baseline screening round, the STAI anxiety test score was significantly higher compared to the baseline score. This outcome occurred even when the significance of a result of this type was clearly explained, with emphasis on the low risk of the individual having cancer.21
An update of the NELSON study data was presented at the 16th WCLC held in Denver (USA) in September 2015.22 Sensitivity, specificity, and positive and negative predictive values of LDCT for the first 3 rounds of screening were superior to those reported in the NLST study. Distribution by stages was also superior in the first 3 screening rounds of the NELSON study compared to the NLST. In the fourth NELSON screening round, performed 2.5 years after the third, the data had worsened significantly, attributed to a longer interval between screening procedures.
Some authors have speculated that exposure to ionizing radiation associated with LDCT screening may cause 1 cancer death for every 2500 participants.23 Others indicate that the risk of developing cancer due to radiation rises to 5–7 cases per 1000 men and 6–13 per 1000 women included in a screening program.24 However, a recent publication from the US Health Physics Society questioned the methodology of these studies and claimed that the risk of cancer attributable to a cumulative dose of less than 50–100mSv is probably very low or non-existent.25 Moreover, technical improvements in modern CT equipment have allowed the dose of radiation to be reduced to 0.2mSv without adversely affecting the quality of the image, while significantly limiting the risk of developing cancer.26 For this reason, then, it is essential to ensure that CT screening programs are conducted with the lowest possible dose. A recent study calculated that doses below the mSv achieved a reduction in the risk attributable to radiation from 8.6 to 0.35 cases per 100000.27
Current Status of the Use of Low-dose Computed TomographyScientific evidence supporting the use of LDCT as a screening tool led the US Preventive Services Task Force (USPSTF) in 2014 to recommend LDCT screening with a 2 B grade of evidence in subjects who met the inclusion criteria for NLST.28 Following the example of other North American scientific societies and cooperative groups that also included this practice among their recommendations,29–33 the U.S. federal and state health insurance programs, Medicare and Medicaid, now include screening among its services.34 The European Society of Radiology (ESR) and the European Respiratory Society (ERS) have published a joint document recommending LC screening with LDCT in centers that have multidisciplinary teams with experience in the diagnosis and management of LC, smoking cessation programs, LDCT equipment (1–3mSv), and the inclusion of healthy volunteers who meet NLST criteria. They also recommend that the screening program is included in a registry or cohort with a proven track record in screening.35
Cost-effectiveness of Low-dose Computed TomographyAn important aspect for implementing any screening strategy is the economic impact and cost-effectiveness of the program. According to NLST data, LDCT screening increases costs by $1631, and provides an additional 0.0316 life years per person and 0.0201 quality-adjusted life years.36 This translates to an incremental cost-effectiveness ratio of $52000 (or $81000 when adjusted for quality), which has been considered acceptable in the United States.37 This outcome can vary widely and depends on the setting and various characteristics of the screened population, such as gender, age, smoking habit and the risk of cancer.
The NLST cost-effectiveness analysis also depended on assumptions that cannot be directly applied to our setting or healthcare system, such as the cost of LDCT, survival according to disease stage, costs associated with surgical treatment, and associated morbidity and mortality. The cost-effectiveness ratio is more favorable in Europe, where healthcare costs are much lower than in the United States. A more favorable cost-effectiveness ratio has been demonstrated by programs such as the UK Lung Cancer Screening (UKLS) trial, in which the selection criteria of patients is based not only on age and smoking history (as in NLST and NELSON), but also on models for estimating individual risk standardized by age, based on factors associated statistically with the risk of developing cancer, such as length of smoking habit, history of pneumonia, personal history of cancer, and exposure to asbestos (Liverpool Lung Project risk model).38
Performing Low-dose Computed TomographyThe American College of Radiology (ACR) and the Society of Thoracic Radiology (STR) have published a joint document that describes the requirements of the equipment, the type of scan, the admissible dose of radiation, the type of structured, standardized report required, and the quality controls needed in an LDCT screening program.39 The ERS/ESR publication also addresses these issues.35,40
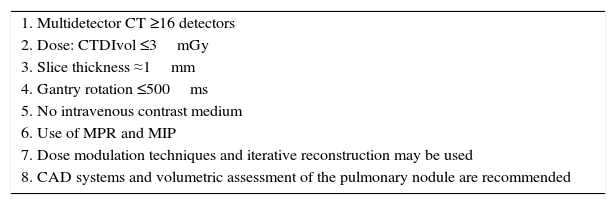
Screening programs must be performed with a CT scanner with at least 16 detectors, and examinations must be carried out at low doses (≤1.5mSv or CT Dose Index Volume [CTDIvol] ≤3mGy). The scanning protocol must be adapted to the body mass index (BMI) of the patient, with a dose of 1mSv recommended for individuals with a BMI corresponding to their ideal weight, and 3mSv for obese individuals. Iterative reconstruction techniques and dose modulation may be used. The examination must be performed without intravenous contrast medium, and the entire lung must be included. Recommended slice thickness is ≤2.5mm and submillimeter slices should be included if possible. Post-processing tools must be available, such as maximum intensity projection (MIP), that increases sensitivity in the detection of nodules, and multiplanar reformation (MPR), that helps to characterize perifissural nodules. It is advisable to use software to help the radiologist in their work, such as computer-aided diagnosis (CAD) systems, using linear and volumetric measurements. If volumetric measurements are used, the same software must always be used. Table 1 summarizes the minimum technical requirements for LDCT in LC screening.
Minimum Requirements for Performing a Low-Dose Computed Tomography Study for Lung Cancer Screening.
| 1. Multidetector CT ≥16 detectors |
| 2. Dose: CTDIvol ≤3mGy |
| 3. Slice thickness ≈1mm |
| 4. Gantry rotation ≤500ms |
| 5. No intravenous contrast medium |
| 6. Use of MPR and MIP |
| 7. Dose modulation techniques and iterative reconstruction may be used |
| 8. CAD systems and volumetric assessment of the pulmonary nodule are recommended |
CAD: computer-aided diagnosis; CTDIvol: computerized tomography dose index volume; CT, computed tomography; MIP: maximum intensity projection; MPR: multiplanar reformation.
A structured, standardized protocol must be used to assess pulmonary nodules and their subsequent management. The NLST criterion that regarded all nodules measuring ≥4mm as a positive finding was associated with a high rate of FPs and is now considered obsolete. New criteria were subsequently developed for the monitoring and intervention of pulmonary nodules that guarantee improved LDCT specificity without affecting sensitivity. The ACR has published some of the most accepted criteria, based on radiological characteristics, called the Lung Imaging Reporting and Data System (Lung-RADS™). According to a retrospective analysis, use of these criteria would have reduced the percentage of FPs in the NLST from the 27.6% published in 2011, to 10%, thus increasing the positive predictive value of LDCT from 6.9% to 17.3%.41,42 The National Comprehensive Cancer Network (NCCN) and the Lung-RADS™ recommend a cut-off point of 6mm for a nodule to be considered of interest; some studies even propose placing the threshold at around 7–8mm, with the consequent reduction in FPs.43,44 Other recent initiatives with the same objective include the Lung Reporting and Data System (LU-RADS)45 and the NELSON study methodology, which analyzes volume-doubling time.17,19
What if a Pulmonary Nodule is Detected?Pulmonary nodules detected by LDCT must be classified using standardized criteria, and monitoring or management must be performed according to a pre-established protocol. One of the most widely accepted protocols, specifically designed for screening programs, is the Lung-RADS™.
The Lung-RADS proposes that indeterminate nodules considered low risk should be monitored in specific assessments determined by their radiological characteristics. Biopsy is reserved for highly suspicious nodules that have a solid component ≥8mm.39,41,44,45
All patients with LC detected during screening will leave the program and will be monitored in line with LC staging and treatment guidelines.46–48
Profile of Individuals to be Included in a Low-dose Computed Tomography Screening ProgramEuropean and North American scientific societies all recommend screening for individuals who meet the NLST inclusion criteria. However, these criteria were questioned by the authors themselves when it was shown that they are fulfilled by only 26.7% of patients with LC in the United States.49 A study conducted by investigators from Pamplona (Spain) and Pittsburgh (USA) suggests that applying the NLST selection criteria limits the benefit of screening. Moreover, the exclusive use of the NLST screening criteria in the Pamplona-IELCAP (P-IELCAP) cohort would have led to a failure to detect 39% of cases of cancer occurring in younger patients or those with lower tobacco exposure.50 A finding of emphysema in the first round of screening may help improve the selection of candidates in subsequent rounds. Selection of candidates for screening on the basis of a combination of NLST criteria and presence of emphysema would have resulted in a higher rate of detection of cases of cancer, with a reduction of up to 52% of cases screened.50 In the future, biomarker analysis might help select patients for screening.
How can we Introduce Screening in our Setting?Despite the accumulated scientific evidence for screening, doubts persist regarding the feasibility of large-scale implementation. In this respect, uncertainty regarding the selection of candidates, the number and frequency of the scans, the management of findings, regional variations and positivity criteria is rife.
This has prompted neighboring countries to undertake initiatives aimed at resolving some of these questions. For example, in the United Kingdom a study similar to NELSON has begun that plans to enroll 32000 individuals using a specific questionnaire to assess individual risk and the management of nodules detected by LDCT.51 In France, a statement has been published advising that screening be performed on an individual basis, with specific recommendations regarding positivity criteria and algorithms for monitoring.52
In Spain, there is a pressing need to evaluate the benefits of implementing a similar initiative. Some screening programs are already in place in Spain, most notably those included in the I-ELCAP program, which have contributed over 10000 participants to this cohort. As the available evidence supports the usefulness of screening for a disease that is as common as it is fatal, failure to act cannot be justified.
The challenges facing LDCT screening in Spain are the following: (a) the divided nature of our National Health System, in which responsibilities are split among the autonomous communities; (b) legal requirements governing population screening (Order SSI/2065/2014)53; and (c) limited resources, infrastructure and personnel.
However, recommendations suitable for our setting can and should be established, including the design of one or more pilot schemes that would allow us to analyze the benefits and risks of screening in our environment. In our opinion, if such a scheme is implemented, a single registry of procedures and findings should be created that would provide crucial information for any proposal involving the implementation of a generalized screening program of the at-risk population in Spain.
The need to reduce smoking is closely related with both the implementation and cost-effectiveness of screening programs. We believe that a feasibility pilot program should be run in association with smoking cessation clinics.54 Dedicated clinics would need to be created, and the initiative would have to be associated with discussions on the funding of smoking cessation initiatives. These clinics could act as a starting points for the selection of candidates for screening, an aspect that would help to rationalize costs.
A special situation is generated by individuals who demand a screening LDCT. Since access to information is easier than ever, a growing demand for LDCT in the healthcare system can be predicted. In view of the evidence, it would be difficult to refuse the procedure if the patient continues to insist after receiving information on the advantages and individual risks. The problem then is where to refer the patient, since carrying out individual, isolated screening does not help to improve the quality of the health system, nor does it generate shared information. It is therefore necessary to create trained multidisciplinary teams who work with standardized technical criteria and a monitoring protocol designed by consensus.
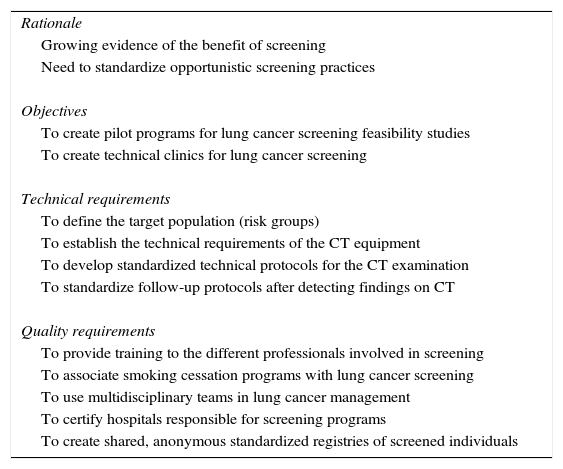
RecommendationsThe recommendations of this group of experts for the implementation of LDCT screening in our setting are the following (Table 2):
- •
Consensus must be reached on the minimum risk criteria for a person to be included in the screening program, the frequency of the procedure, and the number of scans to be performed. The age and smoking criteria used in the NLST are a point of departure: at the time of writing this manuscript, they were the only criteria shown to be associated with a significant reduction in cancer death, and they are currently backed by both the ATS/ACCP and the ERS/ESR. However, we do not need to limit ourselves to these criteria, and they could be modified to limit screening to individuals at greater risk (e.g., those with emphysema).
- •
Positivity criteria for findings must be defined on the basis of diameter or volume of nodules detected, and their subsequent management must be protocolized.
- •
Family and community medicine physicians must be involved in setting up educational campaigns for the LDCT screening program, in initiating parallel smoking cessation activities, and in providing the necessary assistance to achieve and maintain cessation. In addition, as this is not a populational screening program, the collaboration of family doctors seems essential for identifying the selected at-risk population.
- •
Training programs must be set up for participating professionals.
- •
A centralized registry of participants in LDCT screening programs must be created for the analysis of clinical variables, findings, examinations, treatments and their cost, and, finally, progress and survival.
- •
For all these reasons, it would be advisable to select specific, accredited centers in each autonomous community and to audit their activity and outcomes. Feasibility studies of the screening of selected cases would be performed in these centers, and individuals would be seen, studied and monitored by specifically trained multidisciplinary teams, adhering to the established quality standards and the consensus monitoring protocol. The information collected by these multidisciplinary teams would allow for the analysis, within a reasonable period of time, of the real value of implementing an LDCT screening study throughout Spain.
Recommendations for Low-Dose Computed Tomography in Individuals With a High Risk of Developing Lung Cancer.
| Rationale |
| Growing evidence of the benefit of screening |
| Need to standardize opportunistic screening practices |
| Objectives |
| To create pilot programs for lung cancer screening feasibility studies |
| To create technical clinics for lung cancer screening |
| Technical requirements |
| To define the target population (risk groups) |
| To establish the technical requirements of the CT equipment |
| To develop standardized technical protocols for the CT examination |
| To standardize follow-up protocols after detecting findings on CT |
| Quality requirements |
| To provide training to the different professionals involved in screening |
| To associate smoking cessation programs with lung cancer screening |
| To use multidisciplinary teams in lung cancer management |
| To certify hospitals responsible for screening programs |
| To create shared, anonymous standardized registries of screened individuals |
CT: computed tomography.
The authors thank Dr. Fernando Sánchez Barbero for his editorial assistance, and HealthCo for their help in the preparation of this manuscript. We would also like to thank Dr. Javier Zulueta for his contributions to the working group in the initial phases.
Please cite this article as: Garrido P, Sánchez M, Belda Sanchis J, Moreno Mata N, Artal Á, Gayete Á, et al. Reflexiones sobre la implementación del cribado mediante tomografía computarizada de baja dosis en personas con riesgo elevado de padecer cáncer de pulmón en España. Arch Bronconeumol. 2017;53:568–573.