To the Editor: In the past, tuberculosis was considered to be practically the only cause of secondary spontaneous pneumothorax (SSP).1 Currently, few SSPs are attributed to active tuberculosis. Before antibiotics, when techniques such as therapeutic pneumothorax or oleothorax were used to collapse cavitated pulmonary lesions, tuberculous empyema (TE) was relatively common. However, at present, tuberculosis is not considered to be a frequent cause of empyema. We report a case in which a young woman with active tuberculosis developed both these unusual complications: recurrent SSP and TE.
The patient was a 17-year old female with no known relevant medical history. Both parents had suffered tuberculosis 20 years earlier and remained asymptomatic since then. The patient was admitted with cough, dry at first and later producing greenish sputum, temperature as high as 39 ºC, night sweats, asthenia, anorexia, and progressive weight loss in the course of one month. Chest pain was absent. Physical examination revealed slight malnutrition, 39.2 ºC fever, no peripheral lymph node enlargement, and right-sided pulmonary rales.
Laboratory findings upon admission were red blood cell count 3.64x106/µL; hemoglobin 9.20 g/dL; hematocrit 32.60%; white blood cell count 10 100/µL; and platelets 411x103/µL. Biochemical analysis revealed no abnormalities and the chest x-ray showed cavitation in the right hemithorax. A Mantoux test was positive. A test for human immunodeficiency virus was negative. A sputum culture was positive for Mycobacterium tuberculosis. Blood cultures and urine tests were negative for aerobes, anaerobes, and M tuberculosis.
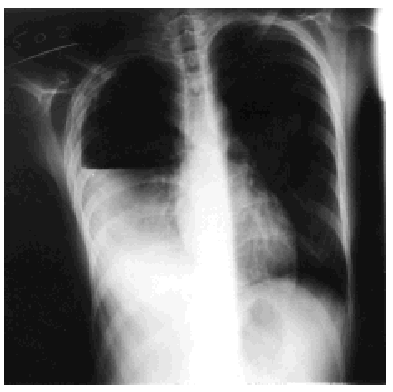
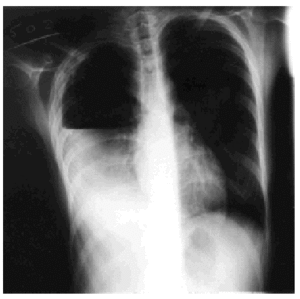
Treatment with rifampicin, isoniazid, and pyrazinamide was commenced upon admission, and sensitivity to all of them was later confirmed. Forty-eight hours after admission, the patient's cough suddenly worsened and increased shortness of breath developed with intense pleuritic pain on the right side. Radiological examination revealed a subtotal pneumothorax of the right lung. A drainage tube was inserted and a yellowish, translucent fluid was removed and found to contain 17.20 leukocytes (80% segmented, 12% with polymorphic nuclei, 0% eosinophils, 8% lymphocytes, and 0% monocytes), and a red blood cell count of 0.01x106/µL. Blood tests revealed pH to be 7.19, glucose 56 mg/dL, total proteins 4.1 g/dL, cholesterol 48 mg/dL, lactate dehydrogenase 9573 U/L, amylase 47 U/L, and adenosine deaminase 82 U/L. A culture of the pleural fluid was positive for M tuberculosis. The chest pain remitted, the pneumothorax resolved, and the drainage tube was removed after 15 days. Fever occasionally as high as 39.5 ºC persisted. Forty-eight hours later the chest pain reappeared and coughing became more frequent, with no increase in expectoration. The x-ray showed a recurrence of pneumothorax, pleural effusion, and right pleural thickening (Figure). A thoracic drainage tube was inserted again and a thick, purulent fluid was removed. Tuberculous aggregates were identified in the Ziehl stain and no concurrent infections were found. A computed tomography (CT) scan of the thorax confirmed the pneumothorax, the loculated pleural effusion, and the pleural thickening. Because of the pleural thickening, the persistence of fever and of empyema, thoracic surgery was considered necessary. A pleural decortication was performed. Visceral and parietal pleural thickening, a posterior empyematic chamber, and a cavity in segment 6 open to the pleural space were detected during the procedure. A few days later the patient was asymptomatic with mild residual pleural thickening and was negative for M tuberculosis within a few weeks.
Figure. Thoracic x-ray: pleural empyema.
In adults, tuberculosis was once a relatively common cause of SSP due to retractions in the pulmonary parenchyma with emphysema and bullae near the pleura arising during the residual fibrosis phase. However, it is considered a very rare complication of active pulmonary infection now, with an incidence of 0.6% to 1.4%.2,3 During fibrosis the most likely pathogenic mechanism is the rupture of the subpleural blebs or bullae. During active infection, and therefore more commonly among young patients, SSP is caused by caseous necrosis with bronchopleural fistula or a cavity open to the pleural space, which could also cause TE. The radiologic expression of this is pyopneumothorax. In our patient, the presence of a cavity beforehand and the absence of increased expectoration, which is more common in patients with bronchopleural fistulae, suggested that the rupture of the cavity due to a cough surge was the most likely cause of both the SSP and the TE. This was confirmed later during the decortication. SSP caused by active tuberculosis seems to appear together with chest pain, cough, and fever more frequently than non-tuberculosis-related SSP. Treatment of patients with SSP caused by active tuberculosis by drainage through a tube with suction is less successful and takes longer.4 However, patients respond favorably to treatment with drugs and drainage tube. This treatment usually reexpands the lung and cures the infection with no recurrence of the pneumothorax.
TE is usually a chronic, active infection located in the pleural space with a large number of tuberculous bacilli. Generally, it arises from a tuberculous fibrous scar, from an artificial pneumothorax, or after a pneumonectomy or a thoracoplasty.5 TE is less frequent than tuberculous pleural effusion. Little is known of the physiopathology of TE in comparison with that of tuberculous pleural effusion (exaggerated inflammatory reaction due to delayed hypersensitivity). TE is caused by an infection of the pleura with M tuberculosis. The clinical signs of TE, which are usually subacute or chronic, are fatigue, fever, chest pain, weight loss, and mainly dry cough, although abundant expectoration will develop later on, as in all cases of tuberculosis or bronchopleural fistula. It is not possible to distinguish empyema from tuberculous pleural effusion through imaging techniques, although the latter involves more encapsulation due to adhesions of pleural surfaces. The presence of fluid and air in chest x-rays in the absence of an evident external cause is indicative of a bronchopleural fistula.6 A CT scan provides the surgical team with a clearer impression of the density of the fluid and of the presence of loculations, and it can establish the existence of pleural thickening. Ultrasound reveals anechoic and hypoechoic images of the parenchyma divided by the visceral pleura. With special transducers, ultrasound can guide a diagnostic thoracocentesis to aspirate fluid or to obtain transthoracic needle biopsy.6 A firm diagnosis is established when a purulent, thick pleural fluid that is positive for acid fast bacilli is obtained by thoracocentesis. The main consequence of TE is pulmonary fibrosis which turns into pleural thickening and later into inability to expand the trapped lung. It also greatly hinders the possibility of treating the pleural space, and that in turn can lead to drug resistance. For this reason evacuation of the empyema is essential. TE must be treated with both antituberculosis chemotherapy and surgery. Indicated surgical procedures can be as simple as the insertion of a drainage tube. However, sometimes more aggressive techniques such as open pleurotomy or thoracotomy are required. The absence of pulmonary expansion, the formation of loculated pleural effusions, and pleural thickening call for thoracotomy to clean and decorticate the cavity and the lung. If a bronchopleural fistula is also present, it must be closed. TE mortality may be as high as 1.3%, by multiple organ failure.