In TNM classification, factors determining the tumor (T) component in non-small cell lung cancer have scarcely changed over time and are still based solely on anatomical features. Our objective was to study the influence of these and other morphopathological factors on survival.
MethodsA total of 263 patients undergoing lung resection due to stage I non-small cell lung cancer ≤3cm in diameter were studied. A survival analysis and competing-risk estimate study was made on the basis of clinical, surgical, and pathological variables using actuarial analysis and accumulative incidence methods, respectively. A risk model was then generated from the results.
ResultsSurvival at 5 and 10 years was 79.8 and 74.3%, respectively. The best prognostic factors were presence of symptoms, smoking habit and FEV1>60%, number of resected nodes>7, squamous histology, absence of vascular invasion, absence of visceral pleural invasion and presence of invasion more proximal than the lobar bronchus. All these were statistically significant according to the actuarial method. The factor “age<50 years” was close to the margin of statistical significance. Pleural invasion and vascular invasion were entered in the multivariate analysis. The competing-risk analysis showed a probability of death due to cancer of 14.3 and 35.1% at 5 and 10 years, respectively. Significant variables in the univariate and multivariate analyses were similar, with the exception of FEV1>60%.
ConclusionsPleural invasion and vascular invasion determine survival or risk of death due to non-small cell lung cancer ≤3cm and can be used for generating a predictive risk model.
En la clasificación TNM, los factores determinantes del factor T en el carcinoma pulmonar no microcítico apenas han variado con el tiempo y todavía se basan únicamente en características anatómicas. Nuestro objetivo fue estudiar la influencia en la supervivencia de estos y otros factores de tipo morfopatológico.
MétodosSe incluyeron 263 pacientes sometidos a resección pulmonar por carcinoma pulmonar no microcítico en estadio I patológico y diámetro ≤3cm. Se realizó un estudio de supervivencia y de estimación del riesgo competitivo observando variables clínicas, quirúrgicas y patológicas, siguiendo los métodos de análisis actuarial y de incidencia acumulativa, respectivamente. Posteriormente, se creó un modelo de riesgo de acuerdo con los resultados.
ResultadosLa supervivencia fue de 79,8 y 74,3% a los 5 y 10 años, respectivamente. Los factores con mejor pronóstico, estadísticamente significativo según el método actuarial fueron: presencia de síntomas, hábito tabáquico, FEV1>60%, número de ganglios resecados >7, tipo histológico escamoso, ausencia de invasión vascular, ausencia de invasión pleural visceral y presencia de invasión bronquial lobar proximal. La edad <50 años rozó la significación estadística. En el análisis multivariante entraron en regresión la invasión pleural visceral y la invasión vascular. El estudio de riesgo competitivo mostró una probabilidad de muerte por cáncer de 14,3 y 35,1% en 5 y 10 años, respectivamente. Las variables significativas en los análisis univariante y multivariante fueron similares excepto el FEV1>60%.
ConclusionesLa presencia de invasión pleural visceral y la invasión vascular determina la supervivencia o el riesgo de muerte por carcinoma pulmonar no microcítico ≤3cm y permiten elaborar un modelo predictivo de riesgo.
The T component of the TNM non-small cell lung cancer (NSCLC) classification system developed by the AJCC and the UICC remained practically the same from 1974 to 2009. One of the changes made in the latest, 7th edition of the classification system undertaken by the IASLC concerns the redefinition of the T factor in stage I. Stage IA is still reserved for tumors ≤3cm with no visceral pleural invasion (VPI) or with no evidence of invasion more proximal than the lobar bronchus (ILB), atelectasis or pneumonitis. However, the introduction of a new 2cm threshold has created 2 new subgroups, T1aN0M0 for tumors ≤2cm, and T1bN0M0 for those measuring between 2.1 and 3cm. Stage IB has also changed in accordance with tumor size: T2aN0M0 now includes tumors measuring ≤3cm with VPI or ILB, or atelectasis or pneumonitis, and also those measuring between 3.1 and 5cm. Tumors measuring between 5.1 and 7cm are reclassified as T2bN0M0, and tumors >7cm as T3N0M0, and included in stage IIB.1,2
Other T descriptor components, such as VPI, ILB, or radiological appearance of the tumor remain unchanged, and the prognostic implications of these elements will be studied in a future review of the classification.3,4
Likewise, other mainly morphological and molecular prognostic factors that could affect survival have been ignored in the TNM classification. Factors such as histologic type, degree of tumor differentiation, vascular invasion (VI), presence of necrosis, etc.,5,6 or molecular factors7 are of interest in establishing prognosis and determining adjuvant therapy options.5,7–9
The purpose of this study is to validate the new approach to staging NSCLC tumors measuring up to 3cm in diameter classified as stage I, and to identify other clinical and morphological prognostic factors not included in the current TNM system. On this basis, we aim to create a risk model for these patients.
Materials and MethodsThe study was conducted from 1 January 1990 to 31 December 2009. The clinical and surgical data from 268 consecutive NSCLC patients with tumor size up to 3cm, classified as TNM stage I, were included prospectively. All patients had undergone anatomical pulmonary resection (lobectomy, bilobectomy or pneumonectomy) with curative intent at the same hospital. Patients undergoing sublobar resection were excluded. For the purpose of this study, samples were analyzed by a single histopathologist.
Five patients that died in the perioperative period were excluded, as the aim of the study was to evaluate the prognostic factors for long-term survival. The remaining 263 patients were followed up for at least 12 months (mean 5.31 years [0.23–21.46]). Follow-up was finalized on 31 December 2010, and the study concluded on 1 January 2011.
Clinical VariablesDemographic variables such as age and sex were analyzed. Age was treated as a continuous variable and dichotomized at 3 cut-off points of 50, 60 and 70 years. The clinical variables included the different symptoms presented at the time of diagnosis, the patient's comorbidity, and their classification according to the Charlson index score.10 Smoking habit was also considered, and patients were classified as smokers, never smokers, and former smokers. Preoperative variables were FEV1 and FVC, expressed as a percentage of predicted value, FEV1/FVC ratio, abnormal ECG findings, and location of the tumor on radiological imaging (right or left lung, lobar, central or peripheral). Fiberoptic bronchoscopy was used to visualize the tumor in the bronchus and the extent of main bronchus involvement.
Surgical variables included the extent of surgical resection, extension to adjacent structures, and the need for angioplasty or bronchoplasty. The number of lymph nodes removed during resection was also analyzed.
Histopathological VariablesTumors were classified histologically and graded according to the WHO 2004 system into 3 groups: high, moderate, or low differentiation. In the case of squamous cell carcinoma, the level of keratinization and the presence of intercell bridges were also assessed. Adenocarcinomas were assessed on the basis of conventional criteria: tumor architecture and atypical cells.
Tumors were measured by their maximum diameter; this parameter was treated as a continuous variable and grouped according to cut-off points of 1 and 2cm. The degree of visceral pleural invasion was assessed according to the system proposed by the IASLC,4 based on the work of Hammar.11 The presence or absence of VI, lymphatic invasion, perineural invasion and tumor necrosis was also determined.
Statistical AnalysisThe study variables were computerized and processed statistically using the RStudio v0.97.320 programming language and environment and the maxstat v0.7-17, survival v2.37-2, Design 2.3-0, prodlim v1.3.7, and cmprsk v2.2-6 packages.
Survival was calculated using Kaplan–Meier actuarial analyses, and results were compared between groups using the log-rank test. Uncensored events were death due to cancer or unknown cause; the latter was assumed to be cancer. Statistical significance was set at P<.05. Variables with a significance level of P<.10 were included in the multivariate time-to-event analysis using the Cox proportional hazard model (incomplete observations).
Individual risk was calculated using the regression coefficients of variables selected in the multivariate analysis. On this basis, patients were allocated to different risk groups according to the presence or absence of prognostic variables. The actuarial curves for these groups were plotted using the Kaplan–Meier method and compared using trend testing. If the results were significant, the survival curves were paired and compared using the log-rank test.
The Kaplan–Meier estimator is the most widely used non-parametric method for calculating survival without the need for the analyzed event to have occurred in all cases (incomplete observation). However, models that take into account risks that compete with the analyzed event, in this case mortality due to causes other than LC, give a more accurate estimate of survival, and can also estimate risk of death from other causes. The cumulative incidence method (competitive risk analysis) meets these requirements, and was therefore chosen to analyze survival in this study.12
For the purpose of cumulative incidence analysis, competing events were death from other causes, including death from a second, metachronous lung tumor. Statistical significance was set at P<.05. Variables with a significance of P<.10 were included in the multivariate analysis performed using the Fine and Gray cumulative incidence model.13
ResultsThe descriptive analysis of patient characteristics and clinical data is shown in Table 1. At the end of the study, 43.7% of patients had survived, 49 (18.6%) had died from causes related to their malignancy, and 6 from unknown causes. In 93 (35.3%) patients, the cause of death was disease other than LC, and in 23 patients, the cause was a second, metachronous tumor. None of the study patients were lost to follow-up (Table 2).
Descriptive Analysis of Study Patients.
| n | % | × | δ | ||
|---|---|---|---|---|---|
| Sex | Men | 226 | 85.9 | ||
| Women | 37 | 14.1 | |||
| Age (years) | 62.5 | 8.8 | |||
| Symptomatology | Asymptomatic | 151 | 57.4 | ||
| Symptomatic | 112 | 42.6 | |||
| Smoking history | Active smoker | 121 | 46.0 | ||
| Former smoker | 108 | 41.1 | |||
| Never smoker | 34 | 12.9 | |||
| Comorbidity | Diabetes mellitus | 20 | 10.6 | ||
| Hypertension | 79 | 30.0 | |||
| Heart disease | 33 | 16.7 | |||
| Peripheral artery disease | 37 | 14.1 | |||
| COPD | 33 | 12.5 | |||
| Cancer | 47 | 17.9 | |||
| Chronic kidney failure | 4 | 1.5 | |||
| Liver failure | 19 | 7.2 | |||
| Charlson index | 0 | 90 | 34.2 | ||
| 1 | 71 | 27.0 | |||
| 2 | 63 | 24.0 | |||
| 3 | 20 | 7.6 | |||
| 4 | 12 | 4.6 | |||
| 5 | 5 | 1.9 | |||
| 6 | 2 | 0.8 | |||
| Spirometry | FEV1/FVC<0.7 | 33 | 12.5 | ||
| FEV1<0.6 | 27 | 10.2 | |||
| ECG | Normal | 233 | 88.6 | ||
| Abnormal | 30 | 11.4 | |||
| Lobe | Right main bronchus | 2 | 0.8 | ||
| Right upper lobe | 89 | 33.8 | |||
| Bronchus intermedius | 4 | 1.5 | |||
| Middle lobe | 13 | 4.9 | |||
| Right lower lobe | 36 | 13.7 | |||
| Left main bronchus | 10 | 3.8 | |||
| Left upper lobe | 79 | 30.1 | |||
| Left lower lobe | 30 | 11.4 | |||
| Thoracotomy | Right side | 144 | 54.8 | ||
| Left side | 119 | 45.2 | |||
| Extent of resection | Lobectomy | 228 | 86.7 | ||
| Bilobectomy | 11 | 4.2 | |||
| Pneumonectomy | 24 | 9.1 | |||
| Typical/atypical | Typical | 256 | 97.3 | ||
| Extended to lobar segment | 3 | 1.1 | |||
| Bronchoplasty | 3 | 1.1 | |||
| Angioplasty | 1 | 0.4 | |||
| Histology | Epidermoid | 115 | 43.7 | ||
| Adenocarcinoma | 134 | 50.9 | |||
| Anaplastic large-cell | 14 | 5.3 | |||
| Tumor size (cm) | 2.2 | 0.7 | |||
| Tumor differentiation | G1 | 123 | 46.8 | ||
| G2 | 92 | 35.0 | |||
| G3 | 48 | 18.2 | |||
| Lymphatic invasion | Yes | 56 | 21.3 | ||
| No | 207 | 78.7 | |||
| Perineural invasion | Yes | 33 | 12.5 | ||
| No | 230 | 87.5 | |||
| Necrosis | Yes | 182 | 69.2 | ||
| No | 81 | 30.8 | |||
| Vascular invasion | Yes | 60 | 22.8 | ||
| No | 203 | 77.2 | |||
| Pleural invasion | p10 | 229 | 87.1 | ||
| p11 | 32 | 12.1 | |||
| p12 | 2 | 0.8 | |||
| T1 | T1a | 72 | 27.4 | ||
| T1b | 105 | 39.9 | |||
| T2a | Bronchial | 42 | 16 | ||
| VPI | 34 | 12.9 | |||
| Fissure | 10 | 3.8 |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity: VPI: visceral pleural invasion.
Causes of Death.
| Local recurrence | 5 | 1.9 |
| Locoregional recurrence | 8 | 3 |
| Distant recurrence | ||
| Central nervous system | 12 | 4.5 |
| Several levels | 11 | 4.1 |
| Bone | 7 | 2.6 |
| Lung | 4 | 1.5 |
| Liver | 1 | 0.3 |
| Suprarenal | 1 | 0.3 |
| Unknown | 6 | 2.3 |
| Cardiovascular | ||
| Acute myocardial infarction | 13 | 5.3 |
| Stroke | 7 | 2.6 |
| Aortic aneurysm | 3 | 1.1 |
| Heart failure | 3 | 1.1 |
| Cor pulmonale | 1 | 0.3 |
| Pulmonary thromboembolism | 1 | 0.3 |
| Intestinal ischemia | 1 | 0.3 |
| Respiratory | ||
| Pneumonia | 10 | 3.8 |
| COPD | 6 | 2.2 |
| Others | ||
| Traffic accident | 2 | 0.7 |
| Urinary sepsis | 1 | 0.3 |
| Terminal kidney failure | 1 | 0.3 |
| Cancer | ||
| Bladder | 5 | 1.9 |
| Bowel | 2 | 0.7 |
| Larynx | 2 | 0.7 |
| Prostate | 2 | 0.7 |
| Amygdala | 1 | 0.3 |
| Hypopharyngeal | 1 | 0.3 |
| Esophageal | 1 | 0.3 |
| Stomach | 1 | 0.3 |
| Tracheal | 1 | 0.3 |
| Liver | 1 | 0.3 |
| Melanoma | 1 | 0.3 |
| Hematologic | 2 | 0.7 |
| Second metachronous LC | 23 | 8.7 |
COPD: chronic obstructive pulmonary disease; LC: lung cancer.
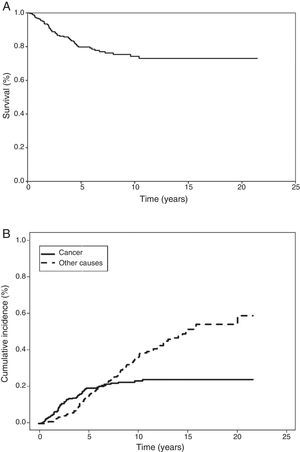
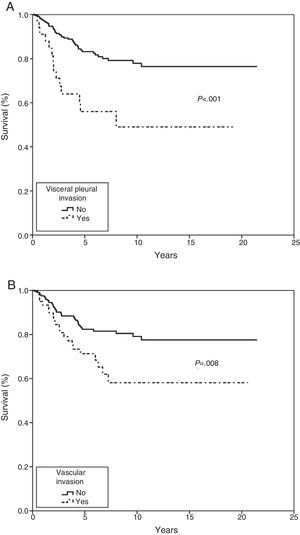
In the Kaplan–Meier actuarial analysis, the cancer-specific probability of survival was 79.8% and 74.3% at 5 and 10 years, respectively (Fig. 1). No difference was observed in terms of the 2cm cut-off point, or in the analysis of the T1 and T2, or T1a, T1b and T2a subgroups. Statistically significant factors for good prognosis were presence of symptoms (P=.040), smoking history (P=.037), FEV1>60% (P=.032) and removal of more than 7 lymph nodes during resection surgery (P=.035). Age<50 years was border-line significant (P=.050). In terms of histological variables, epidermoid tumor (P=.042), absence of VI (P=.008), absence of VPI (P<.001) and ILB (P=.004) were significantly associated with longer survival (Table 3).
Kaplan–Meier Univariate Analysis vs Gray's Cumulative Incidence Method.
| n | 5 a (%) | KM (P) | 1-KM | 5 a (%) | IA (P) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Men | 226 | 80.8 | .370 | 19.2 | 18.2 | .256 |
| Women | 37 | 73.5 | 26.5 | 25.8 | ||
| Age (years) | ||||||
| ≤50 | 21 | 94.4 | .050 | 5.6 | 5.3 | .118 |
| >50 | 242 | 78.4 | 21.6 | 20.3 | ||
| ≤60 | 106 | 80.1 | .754 | 19.9 | 19.0 | .977 |
| >60 | 157 | 79.9 | 20.1 | 19.0 | ||
| ≤70 | 206 | 79.7 | .703 | 20.3 | 19.1 | .795 |
| >70 | 57 | 80.2 | 19.8 | 18.8 | ||
| Symptoms | ||||||
| Asymptomatic | 151 | 77.4 | .040 | 22.6 | 21.6 | .038 |
| Symptomatic | 112 | 83.0 | 17.0 | 15.8 | ||
| Smoking habit | ||||||
| Never | 34 | 74.7 | .064 | 25.3 | 24.9 | .034 |
| Smoking history | 229 | 80.6 | 19.4 | 18.2 | ||
| Never | 34 | 74.7 | .037 | 25.3 | 24.9 | .021 |
| Smoker | 121 | 74.9 | 25.1 | 12.9 | ||
| Ex-smoker | 108 | 86.3 | 13.7 | 22.5 | ||
| Charlson index | ||||||
| 0 | 90 | 80.4 | .641 | 19.6 | 18.5 | .788 |
| >0 | 173 | 79.5 | 20.5 | 19.4 | ||
| ≤2 | 224 | 79.0 | .723 | 21.0 | 20.1 | .929 |
| >2 | 39 | 85.7 | 14.3 | 13.2 | ||
| COPD | ||||||
| FEV1/FVC<70% | 33 | 72.0 | .564 | 28.0 | 24.9 | .692 |
| FEV1/FVC≥70% | 230 | 80.9 | 19.1 | 18.2 | ||
| FEV1<60% | 27 | 58.0 | .032 | 42.0 | 36.1 | .069 |
| FEV1≥60% | 236 | 82.0 | 18.0 | 17.0 | ||
| ECG | ||||||
| Normal | 233 | 80.5 | .640 | 19.5 | 18.45 | .771 |
| Pathological | 30 | 74.5 | 25.5 | 23.84 | ||
| Extent of resection | ||||||
| Partial resection | 239 | 78.7 | .100 | 21.3 | 20.2 | .083 |
| Pneumonectomy | 24 | 90.8 | 9.2 | 8.5 | ||
| Lobectomy | 228 | 78.2 | .126 | 21.8 | 20.6 | .119 |
| Bilobectomy | 11 | 88.9 | 11.1 | 11.1 | ||
| Pneumonectomy | 24 | 90.8 | 9.2 | 8.5 | ||
| Lymph nodes | ||||||
| ≤7 | 212 | 77.3 | .035 | 22.7 | 31.3 | .033 |
| >7 | 51 | 91.9 | 8.1 | 8.0 | ||
| Histology | ||||||
| Squamous | 115 | 84.9 | .113 | 15.1 | 14.5 | .103 |
| Adenocarcinoma | 134 | 75.9 | 24.1 | 22.6 | ||
| Large-cell | 14 | 77.1 | 22.9 | 22.1 | ||
| Squamous | 115 | 84.9 | .042 | 15.1 | 14.5 | .037 |
| Non-squamous | 148 | 76.1 | 23.9 | 22.5 | ||
| Adenocarcinoma | 134 | 75.9 | .036 | 24.1 | 22.6 | .040 |
| Non-adenocarcinoma | 129 | 83.9 | 16.1 | 15.4 | ||
| Tumor size | ||||||
| ≤2cm | 109 | 77.4 | .330 | 22.6 | 20.7 | .381 |
| >2cm | 154 | 81.1 | 18.9 | 18.1 | ||
| ≤1cm | 23 | 80.7 | .827 | 19.3 | 17.6 | .767 |
| >1cm–≤2cm | 86 | 78.4 | 21.6 | 19.9 | ||
| >2cm | 154 | 80.3 | 19.7 | 18.9 | ||
| Tumor differentiation | ||||||
| G1 | 123 | 82.4 | .986 | 17.6 | 16.7 | .980 |
| G2 | 92 | 77.3 | 22.7 | 21.7 | ||
| G3 | 48 | 77.4 | 22.6 | 20.5 | ||
| Lymphatic invasion | ||||||
| No | 207 | 82.0 | .115 | 18.0 | 17.0 | .114 |
| Yes | 56 | 71.9 | 28.1 | 26.7 | ||
| Perineural invasion | ||||||
| No | 230 | 80.7 | .763 | 19.3 | 18.2 | .688 |
| Yes | 33 | 74.0 | 26.0 | 24.9 | ||
| Necrosis | ||||||
| No | 81 | 81.8 | .526 | 18.2 | 17.0 | .596 |
| Yes | 182 | 79.0 | 21.0 | 20.0 | ||
| Fissure invasion | ||||||
| No | 253 | 79.0 | .395 | 21.0 | 19.8 | .427 |
| Yes | 10 | 80.0 | 20.0 | 0.0 | ||
| Vascular invasion | ||||||
| No | 203 | 82.4 | .008 | 17.6 | 16.4 | .007 |
| Yes | 60 | 71.3 | 28.7 | 27.8 | ||
| Pleural invasion | ||||||
| No | 229 | 83.2 | .000 | 16.8 | 15.8 | .000 |
| Yes | 34 | 56.0 | 44.0 | 42.4 | ||
| T factor | ||||||
| T1 | 177 | 82.0 | .332 | 18.0 | 17.0 | .296 |
| T2 | 86 | 75.4 | 24.6 | 23.4 | ||
| T1a | 72 | 80.4 | .400 | 19.6 | 17.9 | .402 |
| T1b | 105 | 82.8 | 17.2 | 16.4 | ||
| T2a | 86 | 75.4 | 24.6 | 23.4 | ||
| T2 (pleural invasion) | 34 | 56.0 | .004 | 44.0 | 42.4 | .003 |
| T2 (proximal bronchial) | 42 | 84.6 | 15.4 | 14.5 | ||
The 1-KM column has been added to facilitate comparison between KM and CI results.
COPD: chronic obstructive pulmonary disease. FVC: forced vital capacity FEV1: forced expiratory volume in 1s; CI: cumulative incidence; KM: Kaplan–Meier.
In the Cox proportional hazard analysis, only 2 variables entered into the regression model: VPI (P=.001) and VI (P=.023) (Table 4 and Fig. 2).
Multivariate Analysis: Cox Proportional Hazard Method vs Fine–Gray Method.
| Multivariate (Cox) | Multivariate (Fine–Gray) | |||||
|---|---|---|---|---|---|---|
| B | P | HR (95% CI) | β | P | HR (95% CI) | |
| Vascular invasion | 0.644 | .023 | 1.90 (1.09–3.32) | 0.628 | .020 | 2.33 (1.10–3.18) |
| Pleural invasion | 1.022 | .001 | 2.77 (1.50–5.13) | 0.962 | .001 | 3.24 (1.46–4.68) |
HR: hazard ratio; CI: confidence interval.
Cumulative incidence analysis, meanwhile, estimated risk of death from cancer as 19.4% and 23.2% at 5 and 10 years, respectively (Fig. 1). When death from other causes was analyzed as a competing event, risk of death was 14.3% and 35.1% at 5 and 10 years, respectively. It is interesting to note that the probability of death from cancer and from non-cancer causes at 6.3 years was the same. Thereafter, the risk of death from non-cancer-related causes increased (Fig. 1).
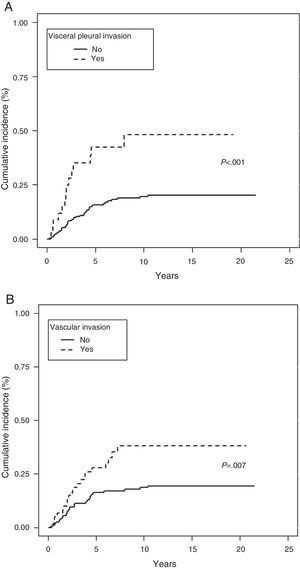
In the analysis of cancer as the cause of death, no differences were observed in terms of tumor size or TNM subgroup, such as T1a, T1b, and T2a. However, presence of symptoms (P=.038), history of smoking (P=.034), removal of more than 7 lymph nodes (P=.033), epidermoid tumor (P=.037), absence of VI (P=.007) or VPI (P<.001) and ILB (P=.003) were protective factors (Table 3).
In the Fine–Gray multivariate analysis, VPI (P=.001) and VI (P=.020) entered into the regression model (Table 4 and Fig. 3).
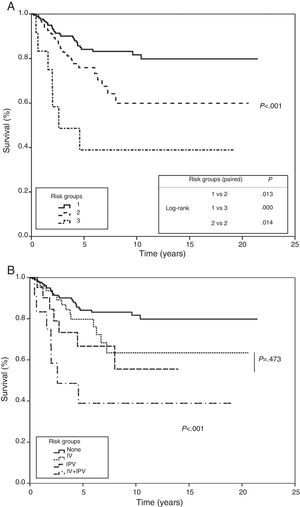
A risk model was created that considered absence or presence, together with the product of their β factor, of each of the 2 variables that entered into the regression analysis in the multivariate analysis. This model was applied to the study population, producing 3 groups: a low-risk group where none of the variables are present, a moderate-risk group, where 1 or other of the variables are present, and a third high-risk group where both are concurrently present. All 3 groups showed statistical significance when they were paired and subjected to trend and log-rank analysis. In the first group, probability of survival at 5 and 10 years was estimated to be 84% and 81%, 75% and 59% in the second group, and 38% in the third (P<.001), respectively (Fig. 4).
Risk groups based on multivariate analysis (Cox proportional hazard method). Survival curves by risk group plotted using significant variables for the multivariate analysis. (A) Group 1: no visceral pleural invasion or vascular invasion; group 2: presence of visceral pleural invasion or vascular invasion; group 3: presence of visceral pleural invasion and vascular invasion. Trend test and comparison of paired curves. (B) Analysis of the intermediate risk group by visceral pleural invasion and vascular invasion.
The TNM system of tumor grading is currently the gold standard for determining treatment and estimating prognosis in NSCLC. However, the involvement of other, non-anatomical factors that could affect survival remains unchanged. In this study, we found that 2 morphopathological variables, IPV and VI, have an independent effect on survival, and can determine a risk prediction model in ≤3cm NSCLC.
In our study, survival at 5 and 10 years was 79.8% and 74.3%, respectively, and echoes the finding of other authors.5,14–16 Although we were unable to compare the results of our cumulative incidence analysis (19.4% and 23.2% risk of death from cancer at 5 and 10 years) with any other series, this analysis allowed us to identify the non-tumor-related factors that compete with LC on a prognostic level, and how, after 6.3 years, these factors prevail over cancer-related risk factors.
Generally speaking, the new TNM classification has been validated with few exceptions.17 However, very few studies in N0M0 tumors with a maximum diameter of 3cm have been published, and the findings so far have been contradictory. Ye et al.18 and Suzuki et al.,19 found that tumors classified as T1aN0M0 had a better prognosis than T1bN0M0 tumors. Li et al.,20 meanwhile, found no differences between these groups or with T2aN0M0 tumors, showing that prognosis was comparable to stage IA. Our results coincide fully with the results of this group, and do not validate the new TNM classification. It is important to note that in our series, 34 tumors that invaded the visceral pleura and therefore classified as T2a had a significantly worse prognosis than 42 other tumors also classified as T2a due to their location in the main bronchus; survival in the latter group was comparable to that of class T1a and T1b tumors.
Interestingly, our study found greater survival in symptomatic patients that were former smokers. The role of smoking as a prognostic factor is unclear, although some studies have also reported improved survival in former smokers.21 Moreover, our finding of poorer survival in asymptomatic patients may be explained by earlier and mostly asymptomatic detection in the last 10 years of the study, and the change in the predominant tumor type to adenocarcinoma, with a poorer prognosis.
Tumor size has been the focus of innumerable studies of the prognostic significance of this factor. In their systematic review, Nesbitt et al.22 found considerable variability in the prognosis of stage I tumors measuring ≤3cm in diameter, and since then several studies have found that prognosis is determined by size.15,23,24 On this basis, the latest TNM classification system set the cut-off point at 2cm. Despite this, other studies echo our findings, namely that size has no impact whatsoever on prognosis.25,26 We did, however, find that it plays a role in competitive risk, since the smaller the tumor, the greater the probability of death from causes other than LC.
ILB is still the criterion for classifying the T descriptor as T2a. Very few studies have explored the prognostic significance of ILB,27,28 and the few references available fail to confirm its impact on survival. This is also confirmed by our results.
The prognostic significance of VPI is controversial, probably due to the lack of a commonly accepted morphological criterion for classifying the extent of the invasion. The IASLC,4 based on studies published by Hammar,11 drew up a series of guidelines to improve the accuracy of comparisons and to identify VPI as an independent factor for poor prognosis in LC9,29,30; our findings are consistent with this recommendation.
In our analysis, VPI was the first variable to enter into the regression model in the multivariate analysis in both the Fine–Gray and Cox proportional hazard model. VPI was also a competitive factor, insofar as absence of this phenomenon determined a greater likelihood of dying from other causes.
In conclusion, we found that tumor size ≤2cm did not determine survival in either of the stage IA groups. Within the T2aN0M0 group, tumors with VPI have poorer prognosis, with a 5-year survival rate of 56%, while survival in IPB tumors was similar to that of T1aN0M0 tumors (84.6%). Nevertheless, competitive risk analysis showed that risk for non-cancer-related mortality in patients classified as T1 and T2 was higher, in the latter case due to bronchial involvement.
With regard to non-anatomical factors, our study of histopathological variables showed that VI had a significant impact on survival. VI was included in both the univariate and multivariate analysis, and was the second factor to enter into regression as an independent prognosis factor in both the Fine–Gray and Cox proportional hazard model.
Although earlier studies on histological factors mention the effect of lymphatic invasion, and not VI, on survival,31,32 more recent research33 confirms our findings, namely, that VI is an independent determinant of survival in stage I tumors. These later studies go so far as to suggest including this factor in the T descriptor of TNM.34 Similar results were reported by other authors who, contrary to our findings, also demonstrated the significance of lymphatic invasion in prognosis.35–37
VI, together with VPI and lymphatic involvement, could even determine the disease-free period in stage I LC.35,38,39
As we have shown in this study, estimation of survival depends on many different factors, and although TNM is still a valuable therapeutic decision-making tool in NSCLC, it does not adequately stratify patients according to prognosis. For this reason we believe the problem should be approached from a multivariate perspective. Developing risk models based on molecular markers would be complicated,7,40 mainly because not all groups have access to the advanced technology needed. However, the problem can also be approached from a clinical and pathological perspective.5,8 Even though our study is limited insofar as we were unable to validate our model in an independent population, we have shown that estimating risk on the basis of VPI extension and VI will identify 3 subgroups of patients with significantly different prognoses. Our findings are largely consistent with those reported by Maeda et al.5,9 who used multivariate analysis to confirm that VPI and VI, together with the degree of tumor differentiation, determined survival in stage I tumors measuring up to 3cm in diameter.
ConclusionsWe believe that the analysis and consideration of clinical and morphological prognosis factors can be put into practice immediately, irrespective of whether or not they are included in future reviews of the TNM classification system. These factors could form the basis for new lines of research into the need for medical treatment as an adjuvant to surgery in early-stage NSCLC, even in small tumors with no signs of extra-pulmonary involvement.
Conflict of InterestThe authors declare they have no conflict of interest.
Please cite this article as: Peñalver Cuesta JC, Jordá Aragón C, Mancheño Franch N, Cerón Navarro JA, de Aguiar Quevedo K, Arrarás Martínez M, et al. Factores pronóstico en el carcinoma bronquial no microcítico menor de 3 centímetros (análisis actuarial, incidencia acumulativa y grupos de riesgo). Arch Bronconeumol. 2015;51:431–439.