Primary pulmonary synovial sarcoma (PPSS) is a rare malignancy that represents less than 0.5% of malignant pulmonary neoplasms and 10% of primary pulmonary sarcomas. It may originate in the pulmonary parenchyma, tracheobronchial tree, pleura, chest wall, mediastinum, or pulmonary artery. However, it does not originate in the synovial membrane, but rather in immature mesenchymal elements.1–4
We report the case of a 67-year-old woman, a smoker, who was admitted for a clinical picture of progressive dyspnea, even at rest, accompanied by constitutional syndrome. Chest radiography revealed extensive right pleural effusion. Chest computed tomography (CT) also showed a solid, heterogeneous, multilobed mass in the lower right lobe, measuring 13cm×9cm×8cm, with partially well-defined contours, and multiple areas of necrosis and hemorrhage, suggestive of primary lung cancer. Thoracentesis yielded a serosanguineous fluid with characteristics of predominantly lymphocytic exudate; cytology was negative for malignancy. Core needle biopsy of the lung mass gave a pathology diagnosis of small cell carcinoma (immunohistochemistry: CD56 positive, TTF-1/STAT6 negative, Ki67 60%) (Fig. 1A). A positron emission tomography-CT confirmed significant pathological uptake by the lesion and the absence of lymph node or distant involvement. Based on these findings, chemotherapy (CT) with carboplatin-etoposide (VP16) was started. After 2 cycles of CT, the patient was admitted for increasing dyspnea and right pleuritic chest pain. Local progression of the right pulmonary mass was observed on imaging tests. Given the disease characteristics (no lymph node involvement or distant metastases, TTF-1 negative) and the atypical course (tumor progression after 2 cycles of CT), the clinical department asked the pathology laboratory to review the case with an expanded immunohistochemical panel. As synovial sarcoma was suspected (weak staining for CD99 and positivity for the EMA), a molecular cytogenetics study by fluorescent in situ hybridization (FISH) for the SYT gene was requested. While awaiting results, the suspicion of synovial sarcoma prompted us to prescribe a new line of CT with epirubicin-ifosfamide, but the ifosfamide had to be discontinued due to encephalopathy and hematologic toxicity. Pathological confirmation was subsequently received of a positive rearrangement for the SYT gene, consistent with a monophasic pulmonary synovial sarcoma, with negative immunohistochemistry for chromogranin/synaptophysin/CD45/CK AE1-AE3, and weak focal positivity for CD99. With this information, CT with adriamycin-cyclophosphamide was prescribed, which also resulted in hematologic toxicity after the first and the second cycle, although a reduction in the solid component of the lung mass was observed in the imaging tests. In view of this evolution, we decided to conduct a right lower lobectomy with parietal pleurectomy. The pathology study of the intraoperative sample (Fig. 1B) confirmed that the tumor was limited to the pleura and did not affect the lung parenchyma. Immunohistochemistry was positive for CD56, WT1 (weak), EMA (focal), and CD99 (weak and focal), and negative for CKAE1/AE3, CK7, CK8/18, CK19, CD34, S100, calretinin, and STAT6.
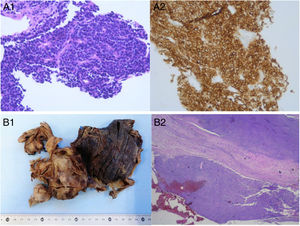
(A) Core needle biopsy of the lung mass: microscopic pathology of the sample, establishing a diagnosis of small cell carcinoma due to the presence of small cells with finely granular oval or spindle-shaped hyperchromatic nuclei, inconspicuous nucleoli, thin nuclear membrane, scant cytoplasm with little staining, scant vascular stroma (A1), and immunohistochemistry positive for CD56 (A2). (B) Intraoperative sample (right lower lobectomy and parietal pleurectomy): macroscopic pathology (B1); overview of the microscopic pathology of the intraoperative sample, showing a tumor confined to the pleura with no lung parenchymal involvement (B2).
PPSS is a very aggressive tumor.1–3 Patients may not have obvious symptoms during the initial stages, but as the disease progresses, dyspnea, chest pain, cough, hemoptysis, and ipsilateral pleural effusion may occur.2,3,5 Chest X-ray and CT generally show a large heterogeneous intrathoracic mass with well-defined margins, measuring more than 5cm,1–3 which often calcifies and invades the pleura.1,3 Necrosis and hemorrhage are almost always seen in the interior of the tumor.2 Primary lung cancer can be indistinguishable from the PPSS on CT. However, the absence of significant lymphadenopathy with a large circumscribed tumor in a young adult may indicate the existence of a PPSS.1,4
From a histological point of view, PPSS is essentially made up of 2 types of cells, epithelial and spindle cells.2 On a histopathological level, PPSS has a broad histological spectrum with 3 variants: a monophasic pattern with spindle cells, which is the most common; a biphasic pattern, with spindle cells and a frequently glandular epithelial component; and a poorly differentiated pattern, with a high mitotic index, small solid oval or spindle-shaped cells with a mixed epithelial and spindle cell appearance, and little differentiation, simulating small cell lung carcinoma. This latter pattern has the most aggressive course and the worst prognosis.2–4
Cytology may be inconclusive.2 Immunohistochemistry is useful for detecting the tumor subtype2: synovial sarcomas are positive for vimentin, TLE-1 and bcl-2 (almost uniformly), CD99 (90%), EMA (55%-91% of cases), cytokeratin (70%) and S-100 (30%),2,3 and negative for desmin, SMA, and vascular tumor markers.4 Cytogenetic tests can be performed when the diagnosis is unclear.3 The presence of the specific chromosomal t (X; 18) (p11.2; q11.2) translocation on FISH is the cytogenetic hallmark of synovial sarcoma. It occurs in more than 90% of cases and causes the fusion of chromosome 18 of the SYT gene with the X chromosome of the SSX1 or SSX2 gene, or more rarely with the SSX4 gene.1,3–5
The prognosis of PPSS is very poor, with a 5-year survival rate after diagnosis of 50%.2,4 The factors for poor prognosis include: tumor size greater than 5cm, male sex, age above 20 years, extensive tumor necrosis, high mitotic rate (more than 10 mitotic figures/10 high-power fields), neurovascular invasion, and presence of SYT-SSXI gene fusion.2,4
Treatment of PPSS should be comprehensive, with surgery, radiation therapy, and CT.6 As with other soft tissue sarcomas, radical surgical resection with sufficiently wide margins is the standard intervention. Adjuvant radiation therapy is usually recommended after incomplete resection. Adjuvant chemotherapy using doxorubicin with or without ifosfamide is beneficial in terms of 5-year disease-free and overall survival in cases of soft tissue sarcoma.7
Please cite this article as: Cerezo Lajas A, Alijo Serrano F, Rodríguez de Guzmán MC, de Miguel Díez J. Sarcoma sinovial pleural primario: diagnóstico diferencial con el carcinoma pulmonar microcítico. Arch Bronconeumol. 2018;54:488–489.