Rigid and semi-rigid thoracoscopy (TS) plays a role in the diagnosis of malignant pleural effusion (MPE), particularly when caused by lung cancer.1–5 The semi-rigid thoracoscope is flexible and less invasive, but it also has some disadvantages: the working channel is narrow, and more importantly, the biopsy forceps is weak and flexible, and obtains smaller samples than those obtained with the rigid thoracoscope. However, its sensitivity and diagnostic accuracy are high,6 and it is also a safe procedure for the diagnosis of unexplained pleural effusion. Additionally, as in rigid thoracoscopy, pleurodesis can be administered in the same procedure.7–10
Molecular characterization of non-small cell lung cancer is gaining importance, tumors that harbor somatic mutations in genes such as the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) will respond better to targeted treatments. Recent years have seen an increase in the number of studies evaluating not only the accuracy of different diagnostic techniques for determining malignancy, but also the adequacy of the samples obtained for immunohistochemical evaluation and molecular determinations of the EGFR mutation and ALK translocation status.11 However, most of these studies focus on endobronchial ultrasound techniques (EBUS-TBNA) and very few data are available on the benefits of TS. Only 1 study has evaluated TS in the analysis of EGFR mutation status, and none has explored ALK translocation in MPE caused by lung adenocarcinoma, probably due to the limited size of the samples obtained with this procedure.12
The aim of this study was to assess the suitability of biopsies obtained by TS as samples for histopathological processing for EGFR and ALK determinations.
We included all patients with MPE associated with lung cancer diagnosed between August 2010 and July 2016 who underwent TS for diagnosis and/or treatment. MPE was defined as an effusion containing malignant cells identified by cytology or pleural biopsy. The study protocol was approved by the local ethics committee, and all patients signed an informed consent form.
The technique was performed in the endoscopy room by a pulmonologist using an ultrasound-guided Olympus LTF-160 thoracoscope with topical anesthesia (2% mepivacaine) and conscious sedation (midazolam and/or fentanyl). Pleural fluid was aspirated while air was allowed to enter the pleural space, which was carefully inspected, and 6-10 biopsies were obtained from the parietal and/or diaphragmatic pleura. Pleurodesis was performed with the instillation of talc via the thoracoscope, if indicated. We recorded thoracoscopic findings, diagnostic yield, and the outcome of the procedure. EGFR mutation status was determined by PCR, and ALK translocation by FISH. Results were expressed as percentages, absolute frequency for qualitative variables, and as mean and interquartile range for numerical variables. Data were analyzed using SPSS version 19.0 (Chicago, IL, USA).
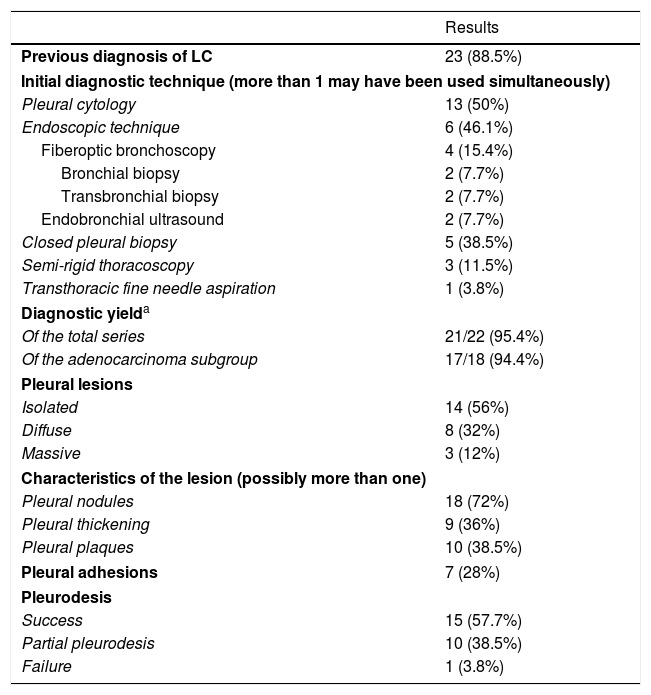
We included our first 26 patients, 69.2% men, with a mean age of 66.5±22.5 years. Seven patients (26.9%) were former smokers, 38.5% (10) active smokers, and 9 (34.6%) never smokers. The most prevalent histological type was adenocarcinoma, in 22 (84.7%) cases, followed by 2 undifferentiated tumors (7.7%), 1 squamous tumor (3.8%), and 1 neuroendocrine tumor (3.8%). Table 1 shows other patient characteristics, diagnostic yield of TS, and findings during the procedure.
Patient Characteristics, Histological Type, Initial Diagnostic Technique, Diagnostic Yield, Findings, and Complications of the Procedure.
| Results | |
|---|---|
| Previous diagnosis of LC | 23 (88.5%) |
| Initial diagnostic technique (more than 1 may have been used simultaneously) | |
| Pleural cytology | 13 (50%) |
| Endoscopic technique | 6 (46.1%) |
| Fiberoptic bronchoscopy | 4 (15.4%) |
| Bronchial biopsy | 2 (7.7%) |
| Transbronchial biopsy | 2 (7.7%) |
| Endobronchial ultrasound | 2 (7.7%) |
| Closed pleural biopsy | 5 (38.5%) |
| Semi-rigid thoracoscopy | 3 (11.5%) |
| Transthoracic fine needle aspiration | 1 (3.8%) |
| Diagnostic yielda | |
| Of the total series | 21/22 (95.4%) |
| Of the adenocarcinoma subgroup | 17/18 (94.4%) |
| Pleural lesions | |
| Isolated | 14 (56%) |
| Diffuse | 8 (32%) |
| Massive | 3 (12%) |
| Characteristics of the lesion (possibly more than one) | |
| Pleural nodules | 18 (72%) |
| Pleural thickening | 9 (36%) |
| Pleural plaques | 10 (38.5%) |
| Pleural adhesions | 7 (28%) |
| Pleurodesis | |
| Success | 15 (57.7%) |
| Partial pleurodesis | 10 (38.5%) |
| Failure | 1 (3.8%) |
LC: lung cancer.
No biopsies were obtained in 4 cases that had been diagnosed previously, since an exclusively therapeutic pleuroscopy was performed due to their functional status and predicted poor tolerance to the procedure. One biopsy showed normal pleura when previous studies had shown it was in reality an adenocarcinoma (the aim of the semi-rigid thoracoscopy in this case was molecular determination).
The oncology department requested molecular testing of 14 adenocarcinomas, and sufficient appropriate material was obtained to determine EGFR mutation status in 100% of cases and ALK translocation in 90% of cases (since insufficient material was available to determine ALK translocation). EGFR mutation was found in 2 patients, and no cases of ALK translocation were identified.
This study shows that obtaining specimens with TS is a very useful procedure for determining EGFR mutation and ALK translocation status in MPE associated with lung cancer. Despite the size of the samples, our results show that they were sufficient to enable a pathologist to correctly interpret and evaluate the molecular mutation status. TS, then, is recommended when previous tests are non-diagnostic, and also when the material is insufficient for histological classification or molecular testing.11 One study showed that the size of the samples obtained by TS was sufficient for pathological subtyping, and that molecular analysis of the EGFR mutation status was possible in 100% of the samples analyzed, although ALK translocations were not included.12 EGFR mutation could be determined in all the cases included in our study, and ALK translocation in all but one. The frequency of both mutations in our sample correlates with the prevalence of both mutations in patients with lung cancer, which is 5%–20% for the EGFR mutation and less than 5% for the ALK translocation.13
One limitation of TS is that the flexible forceps cannot obtain deep samples that include fatty tissue from the wall.12 This is especially important if pleural mesothelioma is suspected. Most findings in our study were pleural nodules or masses, which may increase the diagnostic yield and minimize the possibility of obtaining a falsely negative biopsy.
Although there is a clear distinction between medical thoracoscopy and surgical procedures, considered a good option for diagnosing MPE,14 rigid thoracoscopy under local anesthesia and deep sedation offers the same results in a minimally invasive procedure.3 However, while biopsy samples obtained during TS are smaller than those obtained with rigid forceps, some studies, claim that pleural biopsies obtained by flexible forceps have the same diagnostic potential as those obtained with rigid forceps, in line with the results of our study.15
Our study has certain limitations: the series was small, it was performed in a single center, and most of the patients had undergone previous diagnostic procedures. This is because the objective in patients with lung cancer is to establish the diagnosis, staging, and molecular status with the least invasive technique available.11
Given the growing importance of the identification of mutations, such as the ROS1 proto-oncogene, detection of the induced PDL-PDL1 receptor-ligand pair, and the likelihood of new determinations emerging in the future, new studies must be designed to consolidate the role of TS as a minimally invasive technique that is appropriate for determining these molecular markers.
ConclusionsOur study demonstrates that TS is a minimally invasive technique with a high diagnostic yield that can be used to obtain suitable samples for the detection of EGFR mutations in 100% of cases, and ALK translocations in 90%. It can also be used to perform pleurodesis when deemed necessary.
Please cite this article as: Botana-Rial M, Mouronte-Roibás C, González-Piñeiro A, Fernández-Villar A. Rendimiento diagnóstico de la toracoscopia semirrígida para la caracterización molecular de derrames pleurales malignos de origen pulmonar. Arch Bronconeumol. 2018;54:489–491.