Exacerbations of Chronic Obstructive Pulmonary Disease (ECOPD) are associated with poor clinical outcomes and account for a great proportion of the economic burden of the disease.1,2 In addition, patients hospitalized because of ECOPD have increased risk of future hospital readmissions.3,4 An important proportion of these readmissions occurred early (in the first 90 days) of hospital discharge,5,6 which is related to poor clinical outcomes including higher mortality.7 Prospective research in this area is needed, to identify novel and simple interventions that are effective and implementable at scale.8
The electronic nose (e-nose) is a non-invasive technology that contains an array of electronic chemical sensors capable of identifying volatile organic compounds (VOC) breath-prints.9,10 Some previous studies using e-nose have reported specific breath-prints in patients with COPD,11 asthma,12 diffuse interstitial lung diseases13 and airway infections.14–16 The aim of this study is to investigate whether e-nose can reliably identify a group of patients hospitalized because of ECOPD at risk of early readmission after discharge.
This is a prospective, observational cohort study conducted in a respiratory ward at a university hospital in Barcelona, Spain, that included patients hospitalized due to ECOPD. The sample size needed for this study was estimated on the basis of results previously reported by our group.16,17 Patients were followed-up during 90 days after hospital discharge. Readmission was defined as a new hospital admission due to ECOPD.1 The study protocol was approved by the institutional review board (IIBSP-BRO-2015-92) and all patients signed their informed consent. STROBE guidelines were used to ensure the reporting of this observational study. Exclusion criteria were other respiratory diseases, pneumonia or heart failure at admission. Patients with cancer or a life expectancy of less than 6 months were also excluded. To assess VOC profiles by e-nose, exhaled gas was collected during the first 12h of admission as previously described.12,17,18 In summary, exhaled breath samples of patients admitted for ECOPD were collected in 10l Tedlar bags after 3min of tidal breathing through a Hans-Rudolph valve. The e-nose device (Cyranose 320®; Smith Detections, Pasadena, CA) was then connected to the Tedlar bag for 5min. It requires one minute of purging and 5min of sampling in one-minute periods. The exposure to exhaled breath generated a breath-print VOC profile for each subject. Breath-print data from all participants was analyzed using a pattern recognition application built in the MATLAB software (v.R2012a) as we described previously.14,17 In short, raw data was reduced to three principal factors by principal component analysis (PCA). These PCA factors were used to perform a univariate ANOVA, followed by post-hoc least significant difference test. Patients were then classified into a categorical division using a linear canonical discriminant analysis, calculated as the one that obtained the better percentage of correctly classified subjects. The discriminant function was trained with all minus one subject samples. Then, the remaining samples were tested. This process known as the “leave-one-out” method was repeated for all subjects, thus building the percentage of correctly classified patients which defined cross-validation accuracy values. A Receiver Operating Characteristics (ROC) curve was then generated. The area under the ROC curve (AUC) was calculated with multiple logistic regression, to determine the specificity, sensitivity, positive (PPV) and negative predictive values (NPV) of e-nose to identify patients requiring readmission at 30 or 90 days after discharge. Statistical significance was defined by a 2-tailed p-value <0.05.
A total of 89 patients were included in the study. Most of them (91%) were males with a mean age of 72±9.2 and median FEV1 40% [25–50]. Twenty of them (23%) were current smokers, and hypertension was the most common comorbidity (66%), followed by diabetes mellitus (29%). Seventy-five (84%) patients had taken inhaled corticosteroid (ICS) the previous year and 37 (42%) were on long-term oxygen therapy. On hospital admission, median PaO2 was 56 [50.4–64.1] mmHg, PaCO2 45 [40–54.6] mmHg and C-reactive protein 61 [18.5–130.6] mg/L. Thirty-four (38%) patients had a positive sputum culture for potentially pathogenic microorganisms (PPM), being Pseudomonas aeruginosa the most commonly isolated one (n=19, 56%). After discharge, 17 patients (19%) were readmitted at 30 days and 33 (37%) at 90 days. There were no statistically significant differences in clinical, demographic or treatment variables on admission between patients readmitted or not readmitted at 30 days, except that the readmitted group had higher levels of platelets (307 [242.5–372.5] vs 234.5 [184–297.3]U/ml, p=0.02) and PaCO2 (54 [43.8–62.3] vs 44 [39.3–52]mmHg, p=0.03) at admission. We did not observe any significant difference at baseline between patients readmitted at 90-days or not.
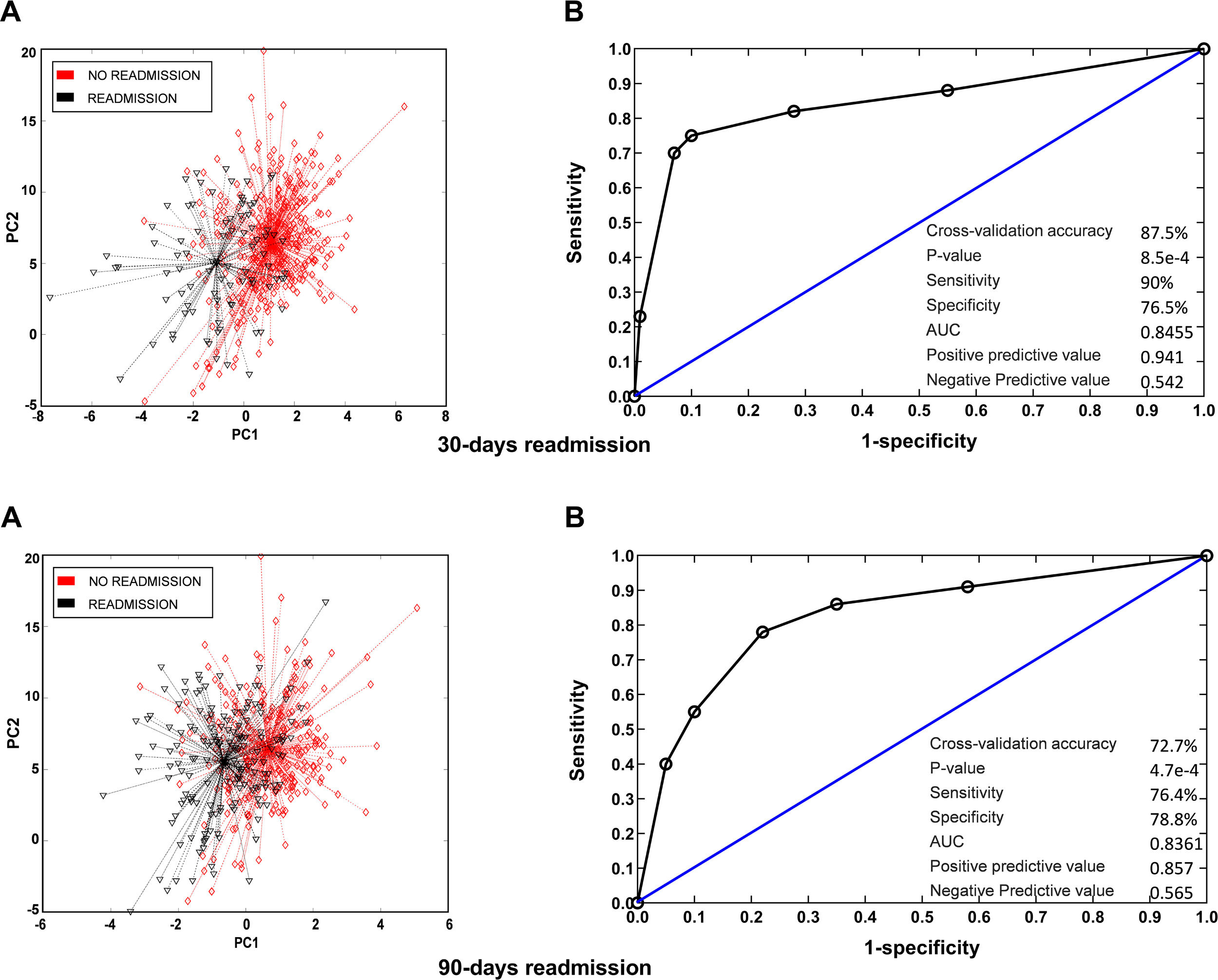
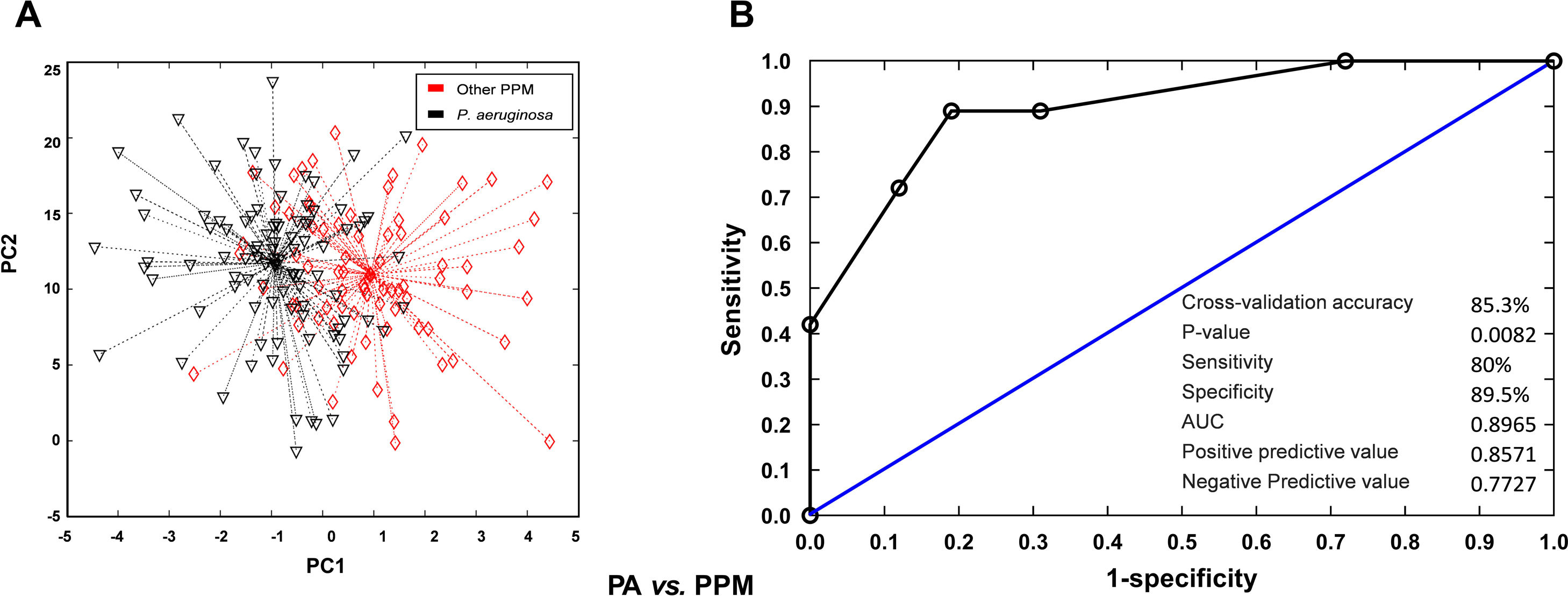
Breath profiles determined by the e-nose at baseline in patients readmitted at 30 days or 90 days were significantly different from those observed in patients who were not readmitted during follow-up. Cross-validated accuracy was 88% at 30 days and 73% at 90 days (p<0.001, both). Sensitivity was 90% at 30 days and 76% at 90 days. Specificity was 77% at 30 days and 79% at 90 days. PPV and NPV was 0.94 and 0.54 at 30 days and 0.86 and 0.57 at 90 days. The AUC was 0.84 for readmissions at 30 days and 0.83 for readmissions at 90 days (Fig. 1). To get further insight into potential variables influencing breath signatures, we perform a subgroup analysis comparing patients according to severity of airflow limitation, smoking status, use of ICS and presence of airway infection on admission. We did not find significant differences in breath-print analyses across these groups except for patients with P. aeruginosa at admission (Fig. 2).
VOC (breathprint) profile in patients readmitted at 30 and 90 days. (A) Principal component analysis showing differences in breathprint between patients readmitted and not readmitted during the follow up. (B) AUROC curve for the discrimination of readmitted patients according to their breathprint.
VOC (breathprint) profile in patients with positive sputum for P. aeruginosa or other PPM. (A) Principal component analysis showing differences in breathprint between patients with positive sputum culture for P. aeruginosa and other PPM. (B) AUROC curve for the discrimination of patients with positive sputum for P. aeruginosa according to their breathprint.
The main finding of this study is that an e-nose can identify accurately ECOPD patients at risk of early hospital readmission. Several previous studies have tried to recognize useful clinical parameters for predicting early readmission of ECOPD patients but, so far, results are conflicting.19,20 Similarly, our study did not show any clinical variable consistently associated with an increased 30-day or 90-day readmission risk. By contrast, e-nose was able to detect differential breath-prints patterns among early readmitted patients (Fig. 1), particularly in those with ECOPD due to P. aeruginosa (Fig. 2). These findings are consistent with previous reports from our group on the performance of e-nose in patients with COPD, both during clinical stability14 and ECOPD episodes.16
E-nose can detect changes in other conditions also characterized by pulmonary inflammation, such as COPD,11 asthma12 and bronchiectasis.14 In fact, Fens et al. showed that exhaled molecular profiles are strongly associated with the type of airway inflammatory cells and their activation status in patients with ECOPD.11 Our result show, for the first time to our knowledge, that an e-nose can be also useful to identify a subgroup of hospitalized ECOPD patients at risk of early readmission after discharge.
Previous studies have demonstrated that P. aeruginosa is associated with a different VOC profile, potentially because specific VOCs may be produced by bacterial metabolism.14 In our study, patients with a positive sputum culture for P. aeruginosa had different VOC breath-prints vs. those infected with other PPM with no differences in other clinical characteristics that may modify VOCs profile.
The major strength of our study is that it tests prospectively a novel and non-invasive diagnostic tool (e-nose) to identify hospitalized ECOPD patients at risk of early hospital readmission after discharge. However, we acknowledge that our study also has limitations. First, although sample size was formally estimated based on results previously reported by our group using the same e-nose,16,17 larger studies are needed to validate our findings. Second, we did not collect airway and blood samples from this cohort of patients to determine inflammatory markers. We plan to include these measurements in future studies using e-nose technology. Finally, we analyze our e-nose data using discriminant analyses, but we did not use gas chromatography or mass spectrometry to study the molecular correspondence of the different VOC patterns determined.
In conclusion, an electronic nose can identify hospitalized ECOPD patients at risk of early readmission after discharge.
FundingThis work have been supported by Sociedad Española de Neumologia y Cirugía Torácica (SEPAR), Societat Catalana de Pneumologia (SOCAP), Fundació Catalana de Pneumologia (FUCAP) and Instituto de Salud Carlos III-Fondos FEDER (PI18/00311).
Conflict of interestNone declared.
Authors thank participants for their willingness to contribute to medical research.