Air leaks are a common problem in thoracic surgery and occur at a rate of 26%–54% on the first postoperative day. Postoperative persistent air leak (PAL) is defined as leak persisting after the third day after surgery according to Brunelli et al.1, after the fourth day according to Cerfolio et al.2, and after the fifth day according to Varela et al.3.
More than 50% of patients undergoing pleural drainage (PD) for pneumothorax develop air leaks at 48 h4. The prevalence of PAL varies between 8% and 20%, and is more common in spontaneous secondary pneumothorax5. Portable devices, such as the Heimlich valve (HV)6, are an accepted strategy in patients with high surgical risk7. HV reduces drainage and hospitalization time5.
The aim of this study was to confirm the safety of HV in patients with postoperative PAL and pneumothorax and to determine its economic benefit.
We performed a descriptive study of a cohort of patients with PD and HV for PAL at hospital discharge between January 2013 and May 2020; the sample size was determined by this time period.
For inclusion, patients had to have PAL after pneumothorax or surgery, they had to be stable, and they had to be independent or have good family support. Exclusion criteria were loss to follow-up, HVs in hospitalized patients, and devices used as a bridge to surgery.
Patients were followed up on an outpatient basis with check-ups every 48–72 h, and PD was withdrawn after radiological revision 24 h after clamping.
The main variables were success rate, proportion of patients with resolved PAL after HV placement, and cost saved per treatment. Epidemiological, clinical, and radiological variables were studied as independent variables.
Mean, standard deviation, and percentiles were calculated for quantitative variables, and frequency and percentages for qualitative variables. The Kolmogorov–Smirnov test was used to determine the normality of the data, the Mann–Whitney U test was used to compare the time distribution between 2 groups, and the Kruskall–Wallis test was used for more than 2 groups. Multiple linear regression with the genetic algorithm technique was used to obtain the most parsimonious model and the bootstrapping technique was used as a non-parametric method. Fisher’s exact test was used to determine the relationship between qualitative variables.
Mean age was 57 ± 17 years; 73% were men. Overall, 69% of PAL cases occurred after thoracic surgery, 34% of which were major pulmonary resections, and 48% were minor; 3% occurred after primary spontaneous pneumothorax, 26% after secondary pneumothorax, and 2% were iatrogenic, for a total of 105 cases in 98 patients.
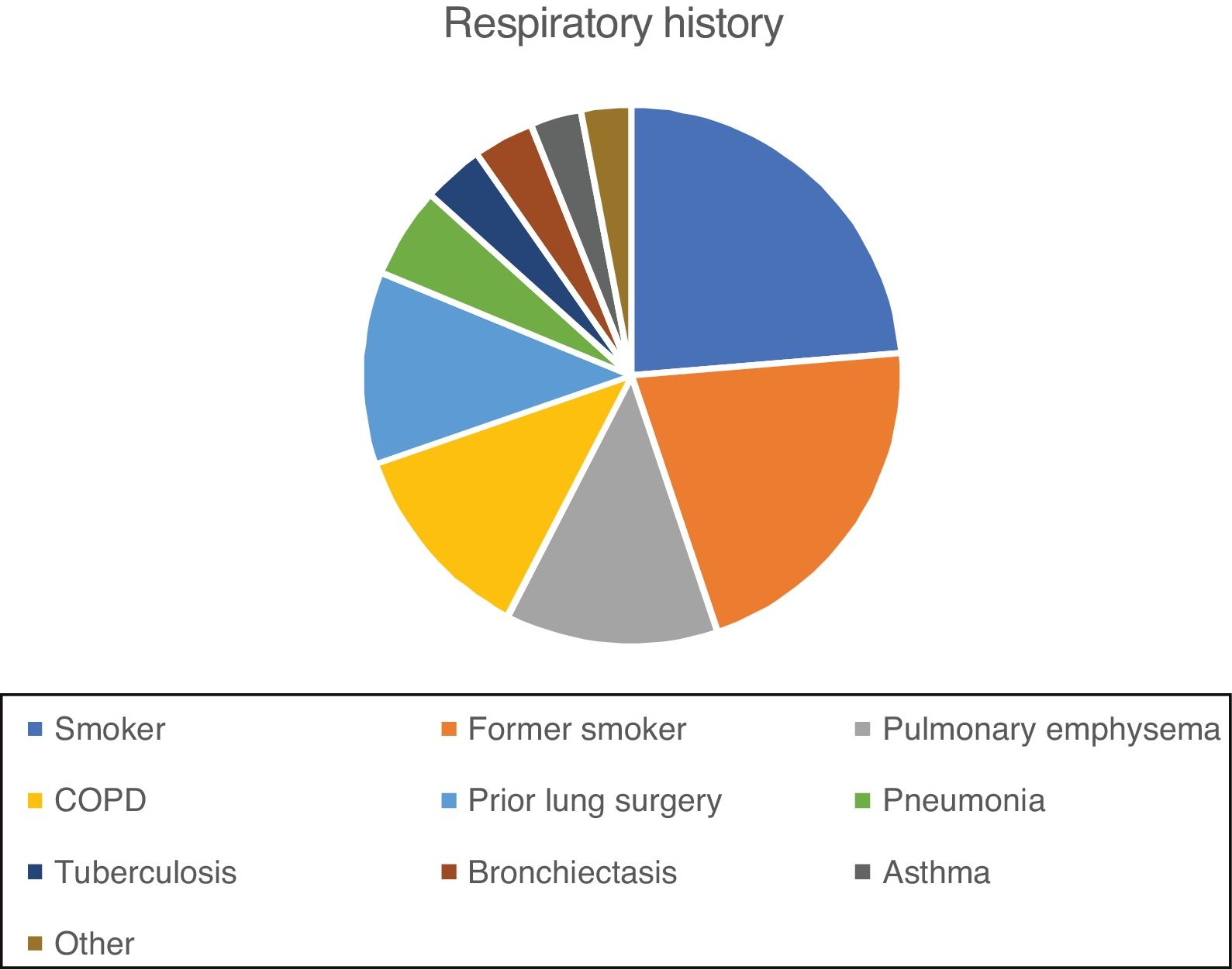
Respiratory history of the series is shown in Fig. 1. Almost one third (30%) of patients had 1 previous event, 30% had 2, 14% had 3, 10% had 4, and 2% had 5.
Overall, 64% of the X-rays prior to HV placement showed an air pocket of less than 20% and 28% showed full expansion of the lung. After withdrawal of the PD and HV, 36% had an air pocket of less than 10%, 44% had lung expansion, and 9.5% had complete pneumothorax. PD was placed for a mean of 16 ± 8 days and HV for a mean of 7.5 ± 5.2 days.
Only 7% of patients had wound infection. Six patients were readmitted, 2 of which resolved when the drainage was connected to an aspiration system and 4 required a new PD (2 received antibiotics for pleural empyema). Another 8 patients were reoperated to resolve PAL. Accidental detachment of the PD occurred in 2 patients.
Since the cost of a room in the thoracic surgery ward in our center is €1453, we calculated a cost saving per treatment of €1,140,605 (785 days).
Multivariate analysis revealed that age, smoking, and tuberculosis were associated with longer PD duration, while smoking, pulmonary emphysema, and tuberculosis were associated with longer HV.
Few studies have been published on outpatient treatment with HV and these consist mainly of retrospective case series in patients with pneumothorax. Our sample size was larger than the mean reported in other studies in the literature (105 vs. a mean of 78.5 cases in the literature)8.
Patients with a history of tuberculosis had a longer duration of PD and longer time to resolution of PAL (p = 0.053). The association of tuberculosis with lung dysfunction and impaired gas exchange9 has been described previously. These factors have been described as predictors of PAL3,10, and are supported by the fact that a history of tuberculosis delays time to resolution of the air leak.
Approximately 90% of cases of PAL are resolved with conservative management and PD placement until air leak resolution11. However, the cost of healthcare increases3 due to the extended hospital stay10,12. Wright et al. described PAL as the second cause of prolonged hospitalization in patients with lobectomy, after pain control12. Varela et al. estimated an excess cost of more than €39,000 for every 6 additional days of hospitalization in 21 patients with PAL3.
Our success rate was 82%, higher than the 77.9% observed in other studies (95% CI: 75.2%–80.4%)8, regardless of etiology.
Alternatives for treating PAL include autologous blood patch pleurodesis13 and other agents14, although these are more invasive and involve potentially serious complications. Endobronchial valves are used in patients who are inoperable or who have failed on conservative treatment15, but these interventions are costly.
Surgery in postoperative PAL is reserved for large leaks and when conservative treatment fails. The technique varies depending on the existing lung damage (simple suture, resections, sealants).
A larger sample size could reveal the influence of factors that have not yet been identified as significant. For this reason, it would be of interest to carry out prospective cooperative studies.
Devices such as HV for the outpatient treatment of PAL offer substantial cost savings for the healthcare system, and complications are minor. Implementation of this strategy may have clinical and economic benefits.
Please cite this article as: Castillo-Acosta S, Castillo-Acosta JC, Rodríguez-Suárez P, González-Martín JM, Freixinet-Gilart JL. Tratamiento ambulatorio de la fuga aérea persistente. Arch Bronconeumol. 2021;57:722–723.