Non-tuberculous mycobacteria (NTM) or atypical mycobacteria are aerobic bacteria of the genus Mycobacterium, the pathogenic potential of which has been known since the 1950s.1 The AIDS pandemic, the progressive increase in immunosuppressive states, and the improvement of microbiological techniques have made isolation of these microorganisms more common nowadays.2
Mycobacterium xenopi (M. xenopi) is a NTM associated with water systems that is found primarily in North America, the south east of Great Britain, and the north of France,3 and was first isolated in immunosuppressed patients. The main risk factors for the disease are chronic lung diseases, during which the organism can colonize the respiratory tract.4 Cases of mycobacterial infection have been published in cancer patients,5 but cases involving M. xenopi are exceptional. No references to the subject were found in articles retrieved from a literature for articles published in Spanish using the standard search engines, Medline and Pubmed (key words: Mycobacterium xenopi and lung cancer). We report a case of M. xenopi infection in a patient with severe COPD and a diagnosis of squamous cell carcinoma.
This was a 73-year-old man, active smoker, diagnosed with severe COPD in 2006. He consulted for asthenia, epigastric pain, weight loss, and productive cough without fever. The only relevant finding on physical examination was poor nutritional status. Clinical laboratory test results showed normochromic normocytic anemia (hemoglobin 10.8g/dl), with ESR 46mm and ferritin 9.4ng/ml (normal value: 30–400ng/ml). Chest radiograph revealed an infiltrate in the left upper lobe (LUL), disperse granulomas, and bilateral air trapping. Gastrointestinal endoscopy showed Candida esophagitis, erosive duodenitis with a negative urease test, and diverticula in the colon. Acid-fast bacilli were observed in 3 sputum samples, so treatment was initiated with isoniazid, rifampicin, pyrazinamide, and ethambutol, in addition to oral iron and fluconazole. After culture of 3 sputum samples in Löwenstein medium grew M. xenopi, the antimicrobial therapy was adjusted, and treatment began with clarithromycin, rifampicin, and ethambutol.
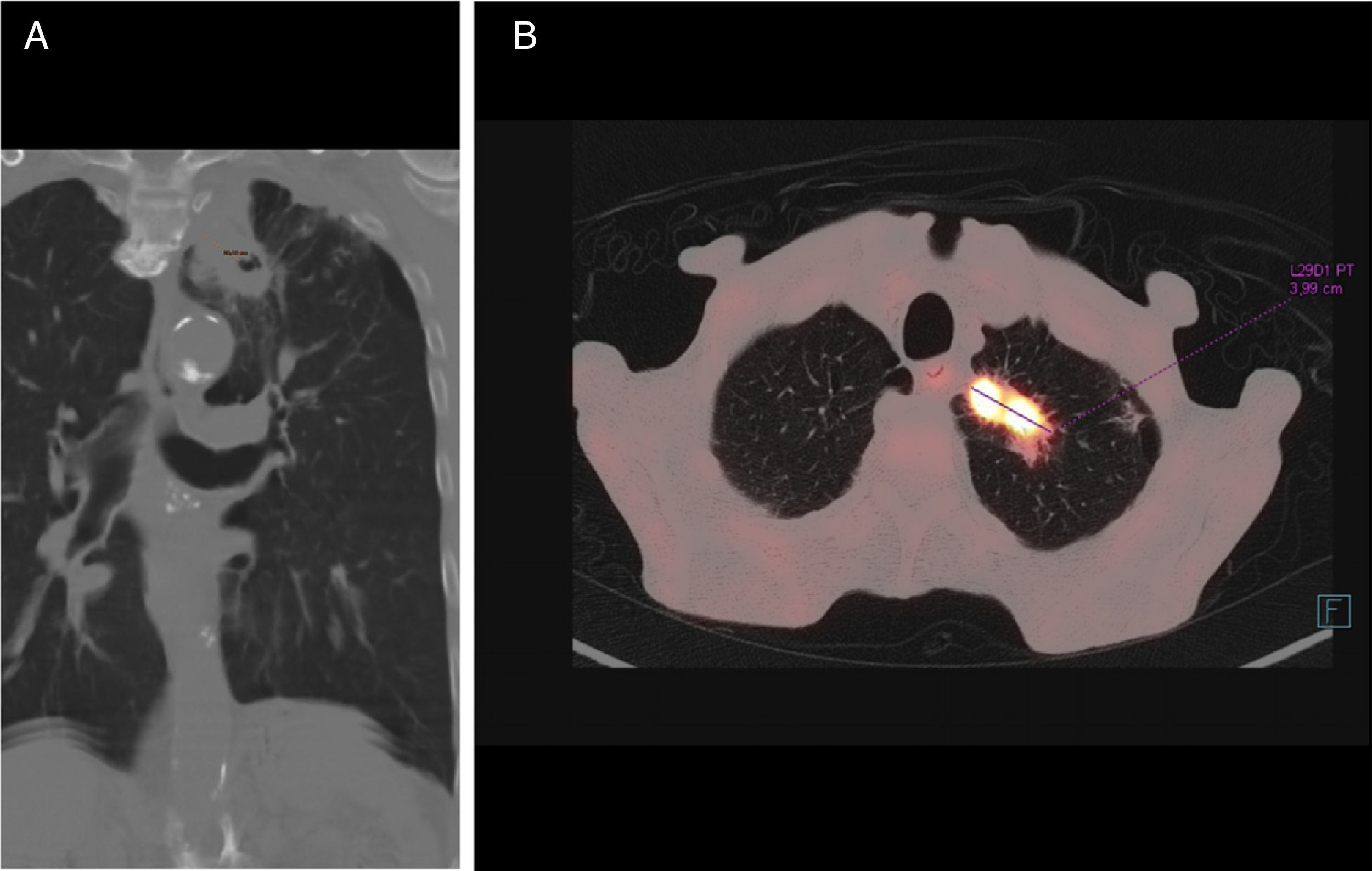
Chest computed tomography (CT) (Fig. 1) confirmed the existence of a solid lesion with a spiculated border and central cavitation in the LUL, measuring 40×35mm, with an 18mm-thick wall, contiguous with another paramediastinal lesion measuring 3cm, signs of emphysema and multiple calcified granulomas. No endoluminal lesions or changes in the mucosa were seen on bronchoscopy. PET-CT confirmed a hypermetabolic mass in the left lung apex, with the appearance of a malignant lung tumor. Transbronchial biopsy yielded a diagnosis of squamous cell carcinoma.
M. xenopi infections usually occur with nonspecific symptoms such as dyspnea, cough, and weight loss,6 and primarily affect males with COPD.7,8 Radiological changes are wide-ranging and usually persistent. Cavitating lesions in the upper lobes, masses, miliary nodules, and mediastinal or hilar adenopathies are common.9 Woodring and Fried10 found that the majority of cavities larger than 15mm in diameter were, as in our case, tumor disease. To our knowledge, 3 cases of M. xenopi infection associated with lung cancer have been published: adenocarcinoma,11 large cell carcinoma,5 and squamous cell carcinoma,12 none of which presented the 2 entities simultaneously, as observed in our case.
To diagnose these diseases, mycobacteria must be grown in 3 sputum cultures, and clinical and radiological evidence must be consistent.13 The best therapeutic combination and optimal treatment duration for M. xenopi lung infections remain to be determined. According to current criteria for NTM infection (ATS/IDSA 2007),8 a 12-month course of a combination of rifampicin, ethambutol, and clarithromycin (or moxifloxacin, due to its low inhibitory concentration against mycobacteria14) is recommended.
Although M. xenopi infection is exceptional, we believe that this case illustrates the importance of ruling out NTM infection in the case of co-existing symptoms or nonspecific signs such as weight loss or anemia in patients with COPD and lung cancer.
Please cite this article as: Martín Asenjo M, Martín Guerra JM, López Pedreira MR, Prieto de Paula JM. Mycobacterium xenopi y carcinoma pulmonar de células escamosas. Arch Bronconeumol. 2017;53:698–700.