We contacted and analyzed the data of 18 lung transplant recipients who had had children. The complications we detected included: hypertension (50%), diabetes mellitus (21%), preeclampsia (13%), infection (21%), rejection (30%), loss of graft function (23%), and a lower percentage of live births than in transplant recipients of other organs.
Other aspects to keep in mind are: the potential risk for fetal alterations (caused by drugs used as prophylaxis against rejection crossing the placental barrier); greater risk for infection and alterations in drug levels due to changes in metabolism typical of pregnancy and postpartum period.
We describe the two cases in Spain of female lung transplant recipients who have had children after transplantation.
Although pregnancy in these cases can have a similar evolution as in non-transplanted women, doctors should recommend their transplanted patients to avoid becoming pregnant, while explaining the high risk of both fetal and maternal morbidity and mortality after transplantation.
Se han notificado 18 trasplantadas de pulmón que han tenido hijos. Las complicaciones detectadas son: hipertensión arterial (50%), diabetes mellitus (21%), preeclampsia (13%), infecciones (21%), rechazo (30%), pérdida de función del injerto (23%) y menor porcentaje de nacidos vivos que en portadoras de otros órganos trasplantados.
Otros aspectos a tener en cuenta son: potencial riesgo de alteraciones fetales dado que los fármacos empleados como profilaxis del rechazo atraviesan la barrera placentaria; así como mayor riesgo de infección y alteraciones de lo niveles de fármacos por los cambios en el metabolismo propios del embarazo y el puerperio.
Se describen los 2 casos en España de mujeres trasplantadas de pulmón que han tenido hijos tras el trasplante.
Aunque el embarazo pueda tener una evolución similar a las que experimentan personas no trasplantadas, se debe recomendar evitarlo y la mujer debe conocer el elevado riesgo de morbimortalidad fetal y materna existente.
Improvements made in the survival, experience, and follow-up of transplanted patients now mean that the desire of many of these patients to become mothers can be made a reality. Since most female transplant recipients maintain their fertility, they may have similar expectations for pregnancy as in the general population.
It is estimated that there have been about 14000 children born to women who have received organ transplants. The immense majority are recipients of kidney or liver grafts, and their reported rates of comorbidities, loss of grafts and neonatal malformations or infections related with immunosuppressant treatment are similar to those of the general population.1–3 The same, however, is not true in the case of lung transplantation recipients.4–7
Described below are the clinical cases of two women who are lung transplant recipients and have given birth to live newborns in Spain.
Case 1: Hospital Marqués de ValdecillaThe patient is currently 31years old and was diagnosed with non-specific interstitial pneumonia (NSIP) in 2002 by lung biopsy. She developed severe respiratory failure despite treatment, and bilateral lung transplantation was performed in May 2003. The patient was treated with tacrolimus, mycophenolate mofetil, and steroids. Her evolution has been satisfactory, and the patient is now asymptomatic and leads a normal life. The lung function has always been normal, with no rejection or infection.
Three years after transplantation, the patient expressed her desire to become a mother, although she was convinced to postpone this decision until 5years after the transplantation. Once the five years had passed, she once again expressed her categorical and intense desire to become a mother. She was informed of the risks, and her immunosuppressant treatment was changed from mycophenolate to azathioprine. Two months later she became pregnant.
The pregnancy was monitored by the Department of Obstetrics and the Transplantation Unit. The pregnancy transpired without complications and lung function was normal at all times. No radiological tests were ordered. Plasma levels of tacrolimus, however, were closely monitored and the dosage was progressively increased from 1.5mg/day to 3.5mg/day in order to maintain plasma levels around 8μg/L. Gestational weight gain was 13kg.
In week 37, and with no apparent reason, there was a strong increase detected in tacrolimus levels (up to 15μg/L). This required the dosage to be quickly reduced to 2mg/day, which was maintained until the end of the pregnancy. After childbirth, the dosage was reduced to 1.5mg/days, which is the same dosage that was used before the pregnancy.
The delivery was normal, in the 40th week of gestation (March 2010) and without complications. The baby was a girl weighing 3.600g, and cord blood levels were 8μg/L, identical to the mother. No anomalies were detected. The newborn only presented mild oliguria that spontaneously resolved itself in 72h. Renal ultrasound was normal.
The baby girl has presented completely normal growth and development. The mother lives a normal life and presents normal lung function. The dosage of tacrolimus that she needs is 1.5mg/day in order to maintain levels at 7μg/L.
Case 2: Hospital Universitario Puerta de HierroThe patient underwent cardiopulmonary transplantation at the age of 38 due to pulmonary hypertension secondary to congenital myocardiopathy with aortic coarctation and ductus arteriosus that had previously been operated on. The patient presented good post-transplantation evolution except, for the development of AHT and digestive intolerance to mycophenolate. Therefore, maintenance of immunosuppression was based on tacrolimus, azathioprine, and corticosteroids.
Seventeen months after transplantation, the patient reported that she had become pregnant despite previous medical advice against it. After being given detailed information about the risks of gestation, the patient decided to continue with the pregnancy. The same immunosuppressant treatment was maintained. At week 16, amniocentesis (with normal karyotype) was done due to the high risk for Down syndrome in the combined first-trimester screening. In the second trimester, the patient presented episodes of diarrhea associated with moderate kidney failure and hypertensive crisis with secondary acute lung edema. During pregnancy, the lung function remained stable and normal with FVC and FEV1 between 90% and 100%.
At week 32, the patient presented with signs of pre-term labor. After completing fetal lung maturation with corticosteroids, and given the limited response to tocolytic treatment, it was decided to perform a cesarean due to the breech presentation of the premature fetus. The newborn presented with resolved myocardial hypertrophy, typical of fetuses exposed to tacrolimus, with later good development and weight gain.
The mother presents normal and stable lung function, and from a cardiologic standpoint she required the placement of a prosthesis in the thoracic descending aorta due to recoarctation, which gave good clinical and hemodynamic results.
DiscussionWorldwide experience about pregnancy and labor in women who have undergone lung transplantation is very limited when compared to the experience seen in other solid organ transplants. Furthermore, the risk of maternal–fetal complications and graft-related problems seems to be higher.
In the 18 patients reported, there have been 24 pregnancies and 15 births. Complications that were detected included AHT (50%), diabetes mellitus (21%), preeclampsia that was difficult to determine due to the presence of AHT and proteinuria prior to the pregnancy in many of these patients (13%), infections during pregnancy (21%), graft rejection (30%) (also in the post-partum) and a significant loss of graft function (23%) during the 2years after childbirth, which is not reported in any other type of transplant.8
The percentage of live newborns was 60%, less than the 70%–80% that is reported in other transplanted organs, with a mean gestation of 35weeks. Most of the newborns (60%) were born with low birth weights (mean 2400g), although no cases of malformation were identified.6,9
All the drugs used as prophylaxis for organ rejection cross the placental barrier. This leads one to think that there must be a certain unquantified risk of interference in the development of the embryo or fetus, which may even be promoted by the use of other medication (antimicrobial prophylaxis, antibiotics, antifungals, antivirals, immunomodulators, bisphosphonates, etc.).10 Azathioprine crosses the placenta as an inactive medicine because the fetus does not have the pyrophosphorylase inositol enzyme, which is necessary to convert azathioprine into its active metabolite. In spite of this, the Food and Drug Administration classifies it as a category D medication. Other antimetabolites, such as mycophenolate mofetil or mycophenolic acid produce a high rate of structural malformations in animals, and cases of fetal death have been reported, so its substitution with azathioprine at least 6weeks before pregnancy has been recommended.11
Some steroids cross the placenta and are metabolized in the placenta itself, so they do not produce significant side effects (category B). There are, however, some doubts about their possible relationship with orofacial malformations.
Anti-calcineurin agents (cyclosporine and tacrolimus) cross the placenta and have an effect on the fetus as in adults, and the possible side effects (renal affectation, hyperglycemia, etc.) have not been able to be quantified. There are doubts about their possible neurocognitive effect. They are used as a basic immunosuppressant treatment (category C).
Other immunosuppressants, such as sirolimus, everolimus, and cyclophosphamide are not recommended due to the evidence of adverse effects and fetal malformations.
In some cases, it is necessary to adjust the dosage of tacrolimus during pregnancy, and there is a case of miscarriage due to incoercible vomiting and difficulty for maintaining the levels of immunosuppression at a therapeutic level.12 Two maternal deaths were reported because they were afraid of the sides effects that the medication may have on the fetus and had stopped taking it.
The time transpired from transplantation to pregnancy should not be less than 3years,13 and it would be better if it were more than 5years. During these years of evolution, the patient should have been stable, with normal lung function, without any important comorbidities or previous acute rejection (unconfirmed data).
In addition to the complications and precautions related with transplanted lungs and their care, it is important to keep in mind the peculiarities of the pregnancy and labor in these patients, such as preeclampsia, whose treatment consists of controlling hypertension with intravenous alpha-methyldopamine and/or labetalol, and the prevention and treatment of convulsions with magnesium sulfate. In those cases with severe maternal/fetal affectation, the definitive treatment would be to terminate the pregnancy.
During labor, it is essential to take the necessary precautions for asepsis, and antibiotics should be used in risk situations (appearance of fever during labor, antibiotic prophylaxis in cases of cesarean, etc.) in order to avoid risk of infection. Another important aspect is immunosuppression in the post-partum. Due to the modifications in metabolism and bioavailability of drugs that the maternal organism goes through after childbirth, it is vital to adjust these levels in order to avoid immunosuppressant toxicity and risk for rejection.
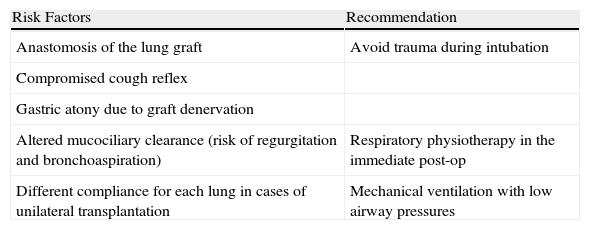
Some considerations have been published about anesthesia during labor (Table 1). Regarding regional analogous anesthesia, neuroaxial blockade techniques (epidural, subarachnoid or combined subarachnoid-epidural) are most widely recommended, both for labor as well as cesarean, because they achieve maximal efficacy for controlling pain and they minimize the sympathetic response that is triggered.14,15 Moreover, they are able to obviate the risks associated with orotracheal intubation, problems associated with mechanical ventilation and the interference between the drugs used during general anesthesia with immunosuppressants. Before performing any type of nerve blockade, it is important to take into account the possible neurotoxicity associated with taking immunosuppressants.
Factors That Increase Anesthesia Risk in Women Who Are Lung Transplant Recipients.
| Risk Factors | Recommendation |
| Anastomosis of the lung graft | Avoid trauma during intubation |
| Compromised cough reflex | |
| Gastric atony due to graft denervation | |
| Altered mucociliary clearance (risk of regurgitation and bronchoaspiration) | Respiratory physiotherapy in the immediate post-op |
| Different compliance for each lung in cases of unilateral transplantation | Mechanical ventilation with low airway pressures |
In these patients with arterial hypotension secondary to the vegetative block of the neuroaxial blockade, afterload should be treated with vasoconstrictor drugs (phenylephrine and ephedrine) due to the greater susceptibility of the transplanted lung for developing acute lung edema due to denervation and the variations of the oncotic pressure in pregnancy.
Immunosuppression makes it necessary to step up aseptic conditions during the placement and handling of the epidural catheter in order to avoid infectious complications.
If general anesthesia is necessary, in the pre-induction phase it is recommended to maximize sterile conditions and administer antibiotic prophylaxis at least 30min before surgery in order to ensure adequate plasma levels. This should be maintained between 24 and 48h post-op.
When the patient is brought out of anesthesia, it is necessary to confirm recovery from the neuromuscular blockade and the consciousness of the patient before extubation.
As for drug interactions, cyclosporine prolongs the duration of the effect of neuromuscular relaxants (in experimental animals). Tacrolimus metabolizes through hepatic cytochrome p450, and therefore caution is recommended with calcium antagonists as they interfere with its metabolism. It is recommended not to use potassium-sparing diuretics.
Although these are isolated cases and definitive conclusions cannot be drawn from them, if the pregnancy is programmed and far in time from the transplantation, the facts demonstrate that, despite the elevated risk of complications, pregnancy after transplantation can be a situation similar to non-transplant recipients. The fetus/newborn may not suffer the consequences of the treatment which the mother is being administered or, if the fetus is affected, the affectation may be mild, reversible, and not interfere with development. Nevertheless, women who are transplant recipients should know about the high risk of fetal and maternal morbidity and mortality that pregnancy entails, and these women should be advised to avoid it.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Zurbano F, et al. Maternidad y trasplante pulmonar: experiencia española. Arch Bronconeumol. 2012;48:379–81.