An excessive risk for bacteremia has recently been reported in patients with pulmonary arterial hypertension (PAH) treated with intravenous treprostinil. We aimed to assess this association in a cohort of patients from a Spanish referral center.
Patients and methodsWe performed a retrospective cohort study that included 55 patients diagnosed with PAH who received a continuous intravenous infusion of a prostanoid (epoprostenol or treprostinil) for ≥1 month at our center between January 1991 and December 2011. The risk factors associated with the incidence of bacteremia were analyzed with the log-rank test.
ResultsAfter a total follow-up of 64453 treatment days, we found 12 episodes of bacteremia: Staphylococcus aureus (5 episodes), non-fermenting gram-negative bacilli (4 episodes), other gram-positive cocci (2 episodes), and Enterobacter cloacae (one episode). The incidence of bacteremia was 0.118 episodes per 1000 treatment days in patients receiving epoprostenol vs 0.938 episodes per 1000 treatment-days in patients receiving treprostinil (P=.0037). All episodes of bacteremia due to Gram-negative bacilli were diagnosed in patients on treprostinil. In the univariate analysis the treatment with intravenous treprostinil was associated with the incidence of bacteremia (hazard ratio: 4.09; 95% confidence interval: 1.24–14.53), although the low number of events prevented us from performing a multivariate analysis.
ConclusionsTherapy with intravenous treprostinil is associated with a higher risk for bacteremia, especially due to non-fermenting Gram-negative bacilli. This association should be taken in consideration when choosing empirical antibiotic therapy for patients with PAH and sepsis.
Recientemente se ha comunicado un exceso de riesgo de bacteriemia en pacientes con hipertensión arterial pulmonar (HAP) que reciben tratamiento con treprostinil intravenoso. Pretendemos evaluar esta asociación en una unidad de referencia española.
Pacientes y métodoEstudio de cohortes retrospectivo con inclusión de 55 pacientes con HAP seguidos en nuestro centro y que recibieron tratamiento mediante perfusión intravenosa continua con un prostanoide (epoprostenol o treprostinil) durante ≥1mes entre enero de 1991 y diciembre de 2011. Analizamos mediante el test de log-rank los factores asociados a la incidencia de bacteriemia.
ResultadosTras un seguimiento total de 64.453 días se documentaron 12 episodios de bacteriemia: Staphylococcus aureus (5 episodios), bacilos gramnegativos (BGN) no fermentadores (4 episodios), otros cocos grampositivos (2 episodios) y Enterobacter cloacae (un episodio). La incidencia de bacteriemia fue de 0,118 episodios por 1.000 días de tratamiento con epoprostenol, frente a 0,938 episodios por 1.000 días de tratamiento con treprostinil (p=0,0037). Todos los casos de bacteriemia por BGN tuvieron lugar en pacientes que recibían treprostinil. En el análisis univariante el tratamiento con treprostinil se asoció a la incidencia de bacteriemia (hazard ratio: 4,09; intervalo de confianza del 95%: 1,24–14,53), si bien el limitado número de eventos impidió la realización de un modelo multivariante.
ConclusionesEl tratamiento con treprostinil intravenoso conlleva un mayor riesgo de bacteriemia, especialmente por BGN no fermentadores. Esta asociación debe ser tenida en cuenta en la elección del tratamiento antibiótico empírico en pacientes con HAP y sepsis.
Thanks to their vasodilator and anti-clotting effects and inhibition of vascular wall remodeling, synthetic prostacyclin analogs (PGI2) are currently included among the first line of therapeutic options in patients with pulmonary arterial hypertension (PAH).1 The two most widely used prostanoids, epoprostenol (Flolan®) and treprostinil (Remodulin®), present short half-lives (3–5min and 2–4h, respectively), which condition their dosage.2 Epoprostenol is an unstable drug at room temperature, and it should therefore be reconstituted with an alkaline diluent (10.2–10.8) before its intravenous (iv) administration by continuous perfusion pump, usually through a central venous catheter (CVC).3 The greater stability of treprostinil allows it to be administered by continuous subcutaneous (sub-cu) perfusion. This drug is presented in the form of sodium salt and should be diluted before perfusion in sterile water or saline solution, maintaining a neutral pH (6.0–7.2).4 Due to the adverse effects at the local level (dolor and inflammation) related with the sub-cu perfusion of treprostinil, in 2004 its administration by IV was approved after having confirmed the bioequivalence between the two formulas.5 In 2007, the US Centers for Disease Control (CDC) observed a unexpected increase in the notification of cases of bacteremia or bloodstream infections in patients who received IV treprostinil, with a particular implication of Gram-negative bacteria (GNB).6 This alarm motivated a retrospective study in 2 reference centers, which confirmed that the incidence of bacteremia due to GNB among patients with PAH being treated with IV treprostinil was significantly higher compared to that of those treated with epoprostenol (0.81 vs 0.04 episodes per 1000 days of treatment, respectively).7 Among other hypotheses, it has been suggested that this finding could be partially justified by differences in catheter maintenance techniques at the participating hospitals or by local epidemiology of nosocomial infection.6 For this reason, we proposed analyzing the incidence of bloodstream and tunnel infections in patients under IV treatment with PGI2 analogs at our center.
Patients and MethodsOurs is a retrospective cohort study with the inclusion of all the patients in follow-up from the PAH Multidisciplinary Unit at the Hospital Universitario 12 de Octubre in Madrid between January 1991 and December 2011 who received continuous perfusion IV treatment with a PGI2 analog (epoprostenol or treprostinil) for at least a month. The PAH Multidisciplinary Unit at our center was the first of its type created in Spain, and it acts as a national reference center. All the patients and their caretakers were instructed in the sterile care of the catheter and the perfusion system, and they were provided with written information as well. Using a standardized form, we collected the following variables: demographic data; comorbidities; PAH etiology; functional class; immunosuppression (infection by human immunodeficiency virus, active neoplasm, or systemic steroid, immunosuppressant or chemotherapy treatment in the previous month); duration of the IV prostanoid treatment; type of catheter used for its perfusion (semi-implantable Hickman CVC, totally implantable CVC [Port-A-Cath], peripherally inserted central catheter [PICC], or peripherally); number of catheters inserted; concomitant administration of other PAH treatments; development of bloodstream or tunnel infections during follow-up, and evolution. We defined “bloodstream infection” as at least one positive blood culture (BC) taken by peripheral venous puncture or through the catheter used for the perfusion. For common saprophyte microorganisms of the skin (coagulase-negative staphylococci [CoNS], diphtheroids, Bacillus spp.), 2 or more positive BC were required, accompanied by symptoms or signs of infection. “Catheter-related bacteremia” (CRB) was considered a bloodstream infection in which the microorganism isolated in the BC coincided with that of the semi-quantitative culture of the tip of the catheter, the peri-insertion skin, the connection or the perfusion liquid, or instead in the presence of symptoms of sepsis in a patient with positive BC with no alternative infection foci. Repeated episodes of documented bacteremia in the same patient that were separated by more than 2 weeks were considered individual episodes. Polymicrobial episodes with isolation of Gram-positive and Gram-negative microorganisms were included in the group with bloodstream infection due to GNB. Tunnel infection was defined based on the presence of signs of local inflammation in the subcutaneous path of the catheter, either with or without purulent exudate at the point of insertion, and in the absence of simultaneous bacteremia.
The qualitative variables are reported with their absolute and relative frequencies; the quantitative variables are given by means or median±standard deviation (SD) or interquartile range (Q1–Q3). The density of the incidence of bloodstream and tunnel infections was calculated per 1000 days of IV treatment with each prostanoid. The comparison between groups was done with the χ2, Fisher's exact, Student's t and Mann–Whitney U tests, as required, with a level of significance set at a P value<.05. Because some patients were treated sequentially with both drugs, two groups were established for comparing their baseline characteristics: epoprostenol group (in whom this was the only analog administered by IV) and the treprostinil group (patients who received this drug, either as the only analog or preceded or followed by treatment with epoprostenol). We divided the study time into 2 periods (1991–2005 and 2006–2011). We estimated the accumulated probability for bloodstream infection from the onset of the treatment with the Kaplan–Meier method, and a univariate analysis was done with the log-rank test. For patients who received both prostanoids sequentially, we analyzed each course of treatment individually. Due to the limited number of events, it was not possible to perform a multivariate analysis. We used the SPSS version 13.0 (SPSS Inc., Chicago, IL) and EPIDAT version 3.1 (Xunta de Galicia/OPS/OMS) application. As the nature of the study was purely observational and collected retrospective data over the course of a lengthy time period, it was not considered necessary to have the Clinical Research Ethics Committee of our center to evaluate the study, nor were specific informed consent forms given to the patients.
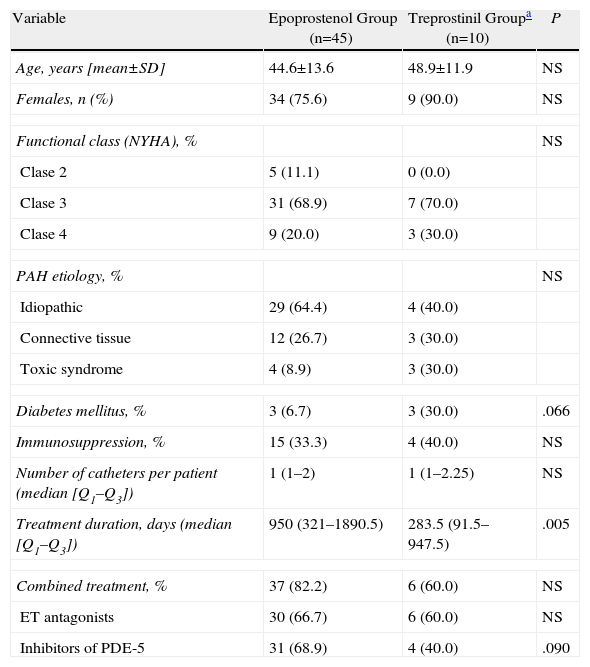
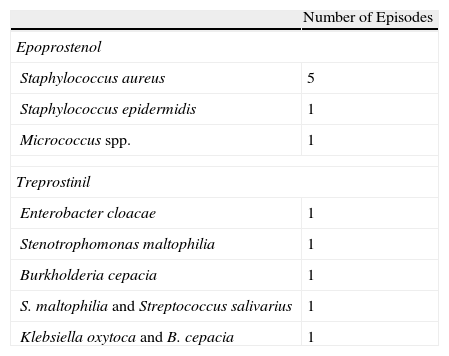
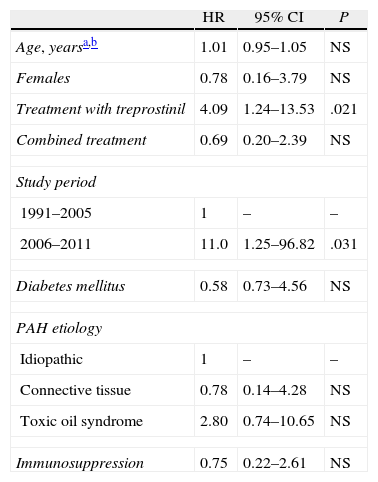
ResultsFifty-five patients were excluded from the study: 45 in the epoprostenol group and 10 in the treprostinil group (2 of whom had also received epoprostenol), with a total follow-up time of 64453 days of IV treatment (59124 and 5329 in each group, respectively). When we compared their baseline characteristics (Table 1), we observed a greater prevalence of diabetes mellitus (DM) in the treprostinil group (P=.066), while the combined treatment with phosphodiesterase 5 inhibitors was more frequent in the epoprostenol group (P=.090). The duration of the IV prostanoid treatment was longer in the epoprostenol group (P=.005). During the follow-up period, there were 11 episodes of tunnel infection, with no differences in their incidence according to the IV prostanoid administered (0.169 vs 0.187 episodes for every 1000 days of treatment in the epoprostenol and treprostinil groups, respectively; P=1.226). The presence of DM was the only variable that demonstrated an association with the development of this complication in the univariate analysis (odds ratio: 6.29; 95% confidence interval [95% CI]: 1.05–37.54; P=.027). A total of 12 episodes of bloodstream infections were documented (global incidence of 0.18 episodes per 1000 days of treatment): 7 in patients who were receiving epoprostenol and 5 in patients who were receiving treprostinil. All the episodes were considered CRB (associated with a Hickman semi-implantable CVC in 6 cases, PICC in 5 cases, and peripheral in one case). The incidence of bloodstream infections was 0.118 episodes per 1000 days of treatment with epoprostenol, vs 0.938 episodes for every 1000 days of treatment with treprostinil (P=.0037). The microorganisms identified were: Staphylococcus aureus (5 episodes), S. epidermidis, Micrococcus spp., Burkholderia cepacia, Stenotrophomonas maltophilia and Enterobacter cloacae (one episode each). The 2 remaining episodes were polymicrobial, with the involvement of GNB (Klebsiella oxytoca and B. cepacia, and S. maltophilia and Streptococcus salivarius, respectively). All the episodes of bloodstream infections due to GNB were diagnosed in patients who were receiving treprostinil i.v. (Table 2). The 2 cases of CRB due to B. cepacia were grouped within a 2-month time period (July and August 2010), and therefore a possible clonal relationship between the two isolations was contemplated, which was later ruled out by pulsed-field gel electrophoresis (PFGE). Fifteen patients (27.3%) died during the study period, although none of the deaths were related with the bloodstream infection. Finally, the administration of treprostinil (hazard ratio [HR]: 4.09; 95% CI: 1.24–14.53; P=.021) and the fact that the patient was treated during the second study period (HR: 11.0; 95% CI: 1.25–96.82; P=.031) were the only factors associated with the incidence of bloodstream infection in the univariate analysis (Table 3).
Baseline Characteristics of the Two Patient Groups According to Intravenous Prostanoid Function.
| Variable | Epoprostenol Group (n=45) | Treprostinil Groupa (n=10) | P |
| Age, years [mean±SD] | 44.6±13.6 | 48.9±11.9 | NS |
| Females, n (%) | 34 (75.6) | 9 (90.0) | NS |
| Functional class (NYHA), % | NS | ||
| Clase 2 | 5 (11.1) | 0 (0.0) | |
| Clase 3 | 31 (68.9) | 7 (70.0) | |
| Clase 4 | 9 (20.0) | 3 (30.0) | |
| PAH etiology, % | NS | ||
| Idiopathic | 29 (64.4) | 4 (40.0) | |
| Connective tissue | 12 (26.7) | 3 (30.0) | |
| Toxic syndrome | 4 (8.9) | 3 (30.0) | |
| Diabetes mellitus, % | 3 (6.7) | 3 (30.0) | .066 |
| Immunosuppression, % | 15 (33.3) | 4 (40.0) | NS |
| Number of catheters per patient (median [Q1–Q3]) | 1 (1–2) | 1 (1–2.25) | NS |
| Treatment duration, days (median [Q1–Q3]) | 950 (321–1890.5) | 283.5 (91.5–947.5) | .005 |
| Combined treatment, % | 37 (82.2) | 6 (60.0) | NS |
| ET antagonists | 30 (66.7) | 6 (60.0) | NS |
| Inhibitors of PDE-5 | 31 (68.9) | 4 (40.0) | .090 |
ET: endothelin receptor; NS: not significant; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PDE-5: phosphodiesterase 5; Q1–Q3: interquartile range; SD: standard deviation.
Distribution of Isolated Microorganisms in the 12 Episodes of Bacteremia.
| Number of Episodes | |
| Epoprostenol | |
| Staphylococcus aureus | 5 |
| Staphylococcus epidermidis | 1 |
| Micrococcus spp. | 1 |
| Treprostinil | |
| Enterobacter cloacae | 1 |
| Stenotrophomonas maltophilia | 1 |
| Burkholderia cepacia | 1 |
| S. maltophilia and Streptococcus salivarius | 1 |
| Klebsiella oxytoca and B. cepacia | 1 |
Univariate Analysis (Log-rank Test) of the Factors Associated With a Greater Accumulated Probability of Bacteremia (Kaplan–Meier Method) in the Course of Treatment With Intravenous Prostanoids.
| HR | 95% CI | P | |
| Age, yearsa,b | 1.01 | 0.95–1.05 | NS |
| Females | 0.78 | 0.16–3.79 | NS |
| Treatment with treprostinil | 4.09 | 1.24–13.53 | .021 |
| Combined treatment | 0.69 | 0.20–2.39 | NS |
| Study period | |||
| 1991–2005 | 1 | – | – |
| 2006–2011 | 11.0 | 1.25–96.82 | .031 |
| Diabetes mellitus | 0.58 | 0.73–4.56 | NS |
| PAH etiology | |||
| Idiopathic | 1 | – | – |
| Connective tissue | 0.78 | 0.14–4.28 | NS |
| Toxic oil syndrome | 2.80 | 0.74–10.65 | NS |
| Immunosuppression | 0.75 | 0.22–2.61 | NS |
95% CI: 95% confidence interval; HR: hazard ratio; NS: not significant; PAH: pulmonary arterial hypertension.
In our experience, the administration of IV treprostinil in patients with PAH was a risk factor for developing CRB, fundamentally to the expense of non-fermenting GNB like S. maltophilia or B. cepacia. The incidence of episodes of bloodstream infections in this group was similar to that reported by the CDC in their original report (1.11 per 1000 days of treatment)6 and by Kallen et al. in a study done in 2 hospitals in the United States (1.13 per 1000 days of treatment).7 The reproduction of these results in series from different centers and countries make it improbable for there to be an influence of local or individual variations in the aseptic manipulation of the catheter or perfusion systems, which is one of the explanations that were initially suggested.6 On the other hand, we did not demonstrate differences in the incidence of tunnel infections between the two groups, which is a complication that could be more directly related to patient compliance with CVC care measures. Thus, other factors must be invoked to justify the excess risk for bacteremia associated with IV treprostinil.
Among these are the differences in the processes of reconstitution and preservation of both drugs in the course of their home administration. Epoprostenol vials are single-dose and, once reconstituted, should be stored at a low temperature (between 2 and 8°C) for a maximum of 24h.3 In contrast, the greater chemical stability of treprostinil allows it to be kept for 48h at room temperature after being diluted in sterile water, and the same vial may be used for repeated perfusions (as long as it does not stay open for more than 30 days).4 The patient loads the perfusion cassette after extracting the drug from the vial, using either a disposable needle or an adaptor that can be used for multiple administrations. There have also been analyses about the pH impact of the diluent used for each drug.8,9 Rich et al. prospectively studied the frequency of bloodstream infections in a group of patients who received treprostinil i.v. reconstituted with the basic diluent of epoprostenol instead of its native diluent (neutral pH).8 No differences were observed in the global incidence of bloodstream infections between this group and those treated with epoprostenol (0.32 vs 0.40 episodes per 1000 days of treatment, respectively), nor in the incidence of bloodstream infections due to GNB. The risk for bacteremia in the intervention group was significantly less in comparison with that of a historical cohort of patients treated with IV treprostinil in its original diluent (0.90 episodes per 1000 days of treatment).8 There is also in vitro evidence that the microbicide activity against Escherichia coli and Pseudomonas aeruginosa of treprostinil reconstituted with the diluent of the epoprostenol is greater than that observed with treprostinil reconstituted in saline solution.9
Pseudomonas spp. was the most frequent GNB in the cases of bloodstream infections in patients treated with IV treprostinil in the series by Kallen et al. (13% of isolations)7 and the CDC (19%).6 Contrarily, the predominant GNB in our cohort were S. maltophilia and B. cepacia, each of them identified in 2 of the 5 episodes of bacteremia in the treprostinil group. It is interesting to indicate that, by using IV treprostinil with the basic diluent of epoprostenol in the study by Rich et al.,8 the Gram-positive cocci were the predominant microorganisms (with isolation of S. aureus in 3–4 episodes), as occurs in the etiology of CRB in the general population. The majority of the CRB produced by B. cepacia complex take place in the context of nosocomial outbreaks due to contamination of IV-administered fluids or antiseptic solutions.10 In our case, we were able to rule out the common origin of both episodes by means of a clonal analysis by PFGE. S. maltophilia has been recognized as an emerging cause of CRB, particularly in oncology patients who are bearers of a permanent CVC.11 The relevance of these non-fermenting GNB is notable due to the fact that their resistance profile requires the use of antibiotics that are usually not included in the empirical treatment of CRB. Co-trimoxazole or tigecycline is active against most B. cepacia strains, so that S. maltophilia accumulates numerous resistance mechanisms, among which are the presence of 2 inducible chromosomal beta-lactamases, types A and B (metalloenzyme) that confer a high level of resistance to β-lactams.11 Co-trimoxazole, in monotherapy or associated with another drug, is considered a first-choice treatment,11 and there is favorable clinical experience with other agents, such as minocycline, tigecycline, ticarcillin/clavulanic acid or quinolones.11,12
With regards to the epoprostenol group, the incidence of bloodstream infections in our study (0.118 episodes per 1000 days of treatment) was lower than that reported by Kallen et al. (0.42 per 1000 days)7 and Rich et al. (0.40 per 1000 days).8 As in this latter, in our series the most commonly isolated microorganism was S. aureus (identified in 5 out of the 7 episodes associated with epoprostenol), in contrast with the predominance of CoNS published by the CDC.6 We did not find any death directly attributable to bacteremia in either of the 2 groups, compared with the mortality of 1.4% from the original US study (with 2 deaths in the patient group that received IV treprostinil).6
Our study is limited by its relatively small sample size, although it was carried out in one of the centers with the longest experience in PAH treatment in Spain. Particularly, the small number of events (12 episodes of bacteremia) impeded the identification (using a multivariate analysis) of the factors that were independently related with the development of this complication. The follow-up period of both groups was not comparable due to the more recent introduction of the i.v. formula of treprostinil in standard practice. In this direction, the univariate analysis revealed that the patients treated during the second study period (2006–2011) were exposed to a greater incidence of bacteremia. Marcos et al. have recently communicated an increase in the incidence of CRB due to GNB in its institution over the course of recent decades (which increased from 0.005 to 0.13 episodes per 1000 days/patient between 1991 and 2008).13 Unfortunately, the absence of a multivariate analysis does not allow us to confirm whether this finding in our study actually the result of historical changes in the epidemiology of the infection associated with sanitary measures, or whether it is the effect of confounding factors that were not considered. In spite of its limitations, to our knowledge the present study is the first to specifically analyze the incidence and the risk factors for bloodstream infection in patients with PAH in Spain and it joins the limited literature that exists on this topic, which until now has been restricted to centers in the United States.6,7
In conclusion, patients with PAH who are treated with IV treprostinil are exposed to a greater incidence of CRB, especially due to non-fermenting GNB. This association should be taken into account when selecting an empirical treatment, given the high mortality associated with the delayed start of effective antibiotic therapy for sepsis in patients with limited hemodynamic reserve. Moreover, it seems reasonable to carefully weigh the benefits and risks of treatment with IV treprostinil, and either use the drug with the basic diluent of epoprostenol or avoid this method of administration in patients who are seniors, have a poor functional class or are immunosuppressed, in whom there would be a poorer prognosis in the event of a bloodstream infection.
FundingFLM has received a research grant from the Mutua Madrileña Foundation. MFR has a Río Hortega Research Training Contract (CM11/00187) at the Carlos III Health Institute.
Conflict of InterestsThe authors declare having no conflict of interests.
The authors would like to acknowledge the work done by the nursing staff of the Pulmonary Hypertension Multidisciplinary Unit (Fernando Romero and Asunción Parra) in the sanitary education and training of our patients.
Please cite this article as: López-Medrano F, et al. Alta incidencia de bacteriemia por bacilos gramnegativos en pacientes con hipertensión pulmonar tratados con treprostinil por vía intravenosa. Arch Bronconeumol. 2012;48:443‐7.
This study was partially presented at the 51st Annual Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, Illinois (September 17–20, 2011) [poster K-842].