Several guidelines recommend computed tomography scans for populations with high-risk for lung cancer. The number of individuals evaluated for peripheral pulmonary lesions (PPL) will probably increase, and with it non-surgical biopsies. Associating a guidance method with a target confirmation technique has been shown to achieve the highest diagnostic yield, but the utility of bronchoscopy with radial probe endobronchial ultrasound using fluoroscopy as guidance without a guide sheath has not been reported.
MethodsWe conducted a retrospective analysis of bronchoscopy with radial probe endobronchial ultrasound using fluoroscopy procedures for the investigation of PPL performed by experienced bronchoscopists with no specific previous training in this particular technique. Operator learning curves and radiological predictors were assessed for all consecutive patients examined during the first year of application of the technique.
ResultsFifty-one PPL were investigated. Diagnostic yield and visualization yield were 72.5% and 82.3% respectively. The diagnostic yield was 64.0% for PPL≤20mm, and 80.8% for PPL>20mm. No false-positive results were recorded. The learning curve of all diagnostic tools showed a DY of 72.7% for the first sub-group of patients, 81.8% for the second, 72.7% for the third, and 81.8% for the last.
ConclusionBronchoscopy with radial probe endobronchial ultrasound using fluoroscopy as guidance is safe and simple to perform, even without specific prior training, and diagnostic yield is high for PPL> and ≤20mm. Based on these findings, this method could be introduced as a first-line procedure for the investigation of PPL, particularly in centers with limited resources.
Varios protocolos recomiendan la tomografía computerizada para las poblaciones con alto riesgo de cáncer de pulmón. Probablemente aumentará el número de sujetos estudiados por lesiones pulmonares periféricas (LPP) y, por lo tanto, también el número de biopsias no quirúrgicas. Se ha demostrado la importancia de asociar un método guía con una técnica de confirmación de la diana para conseguir los máximos rendimientos diagnósticos. Sin embargo, no hay estudios de broncoscopia mediante ecografía endobronquial con sonda radial mediante fluoroscopia, sin vaina guía.
MétodosRealizamos un análisis retrospectivo de los procedimientos mediante ecografía endobronquial con sonda radial mediante fluoroscopia realizados para estudiar las LPP por broncoscopistas expertos pero sin formación previa en esta técnica concreta. Se evaluaron las curvas de aprendizaje de los operarios y los factores pronósticos radiológicos de todos los pacientes consecutivos estudiados durante el primer año de uso de la técnica.
ResultadosSe estudiaron 51 LPP. El rendimiento diagnóstico y el de visualización fueron 72,5 y 82,3% respectivamente. El rendimiento diagnóstico fue del 64,0% para las LPP ≤ 20mm, y del 80,8% para las LPP > 20mm. No se registraron falsos positivos. La curva de aprendizaje de todas las herramientas diagnósticas mostró un RD del 72,7% para el primer subgrupo de pacientes, 81,8% para el segundo, 72,7% para el tercero, y 81,8% para el último.
ConclusiónLa broncoscopia con ecografía endobronquial con sonda radial mediante fluoroscopia es un método seguro y fácil de usar, incluso sin formación específica previa. Su rendimiento diagnóstico es alto tanto para LPP > 20mm como para LPP ≤ 20mm. A partir de estos hallazgos, este método podría presentarse como procedimiento de primera línea para el estudio de las LPP, en especial en centros con recursos limitados.
Lung cancer remains the leading cause of cancer-related death worldwide.1–3 Prevention is the cornerstone of lung cancer control, whereas interventions leading to early diagnosis and stage shift may be the key to a significant reduction in lung cancer mortality. In spite of ongoing controversy on the utility of screening tests, several associations, such as the American College of Chest Physicians, recommend low-dose computed tomography (CT) scan for “high-risk populations”.4–7 As a result, more patients are expected to be evaluated for peripheral pulmonary lesions (PPL), stressing the need for a simple, efficient and safe diagnostic intervention sequence.
Pre-test probability assessment tools are currently used to integrate patient risk factors and the radiological characteristics of nodules to estimate the probability of malignancy and to determine further evaluations or follow-up. These tools, when combined with an evaluation of the patient's surgical risk, can identify several patients requiring non-surgical sampling.4 The latter is indicated as a first-line diagnostic procedure in cases with a low clinical probability for malignancy, in patients with high surgical risk, and in cases with inconsistent clinical and radiological findings.4 For the diagnosis of PPL>8mm, bronchoscopy with endobronchial ultrasound (B-EBUS) competes with transthoracic biopsy (TTB). Both techniques have a good diagnostic yield (DY), but TTB was associated with more complications in a prospective trial.8 Additionally, bronchoscopy allows an extensive evaluation of proximal airways leading to the discovery of synchronous cancers in up to 12% of cases, and also assesses surgical resectability.9
The combination of guidance techniques (referring to navigation in the bronchial tree) and target confirmation techniques (referring to lesion visualization) has been proven to increase the DY of bronchoscopy.10,11 In the case of R-EBUS, all recently published studies combined both guide sheath (GS) and fluoroscopy (F), and reported a DY of 67%–82%.12–14 However, the use of GS requires additional manipulation, potentially increasing the duration of the procedure, and carries an additional economic burden (€204–€276 for Olympus products). The aim of the study was to evaluate fluoroscopic-guided R-EBUS (F-R-EBUS) in PLL sampling without the concomitant use of a GS and without bronchoscopic distance measurements.15–16 For the purpose of this study, safety and efficacy of F-R-EBUS were assessed, learning curves were depicted, and radiological predictors for both visualization yield (VY) and DY were evaluated.
MethodsStudy ParticipantsPatients with PLL evaluated in the University Hospital of Lausanne between November 2011 and November 2012 were eligible for inclusion in the analysis. Patients with a contraindication for bronchoscopy or transbronchial biopsy (TBB) and those with a macroscopically visible endobronchial lesion were excluded. Subjects with associated mediastinal lymphadenopathy on chest CT or on PET-CT scan were first evaluated with mediastinal EBUS, and participated in the study only when the cytology of the lymph nodes was negative. All subjects provided informed consent before bronchoscopy. Retrospective access to clinical data was approved by the Human Research Ethics Committee of the Canton de Vaud (protocol 37/14).
Materials and TechniquesThe procedures were performed under moderate sedation (propofol or midazolam) in an outpatient setting by two experienced bronchoscopists with no prior specific training for F-R-EBUS. During F-R-EBUS, an ultrathin 1.7mm radial ultrasonic probe operating at a frequency of 20MHz (UM-S20-17S Olympus) was used. The ultrasonic probe was introduced in the 2.1mm operating channel of a 4.9mm flexible bronchoscope (Olympus BFP180) without a GS. Antero-posterior fluoroscopy plane was used for guidance.
In case of fluoroscopically visible PPL, the corresponding bronchial segments were sequentially examined until the characteristic ultrasound signal located the lesion. The ultrasonic probe was then removed and the sampling tools were introduced through the identified bronchus. The biopsy forceps was advanced under fluoroscopy guidance until the point where the distal end of the probe was previously visualized. When PPL was not visible by fluoroscopy, the suspected area was located by careful reading of the CT scan, and once the area was reached, all the accessible bronchi were scanned by EBUS until the characteristic signal confirming the site was found. This exact point was visualized by fluoroscopy, the sampling tools were introduced in the identified bronchus, and samples were obtained as follows: transbronchial brush was used in most cases (at least three samplings), TBB (3–5 samplings) only for ultrasound visible lesions, and fine needle aspiration (FNA) (1–3 samplings) for PPL adjacent to the EBUS signal. Bronchoalveolar lavage (BAL) was conducted at the end of the procedure, depending on bronchus collapsibility. Bronchial washing (BW) and BAL were systematically cultured for bacteria, mycobacteria and fungi. In the case of TBB or FNA, a chest X-ray was performed one hour after the procedure to exclude pneumothorax. Diagnosis of malignancy was established by the presence of atypical cells, cells suspected of malignancy, or NSCLC cells in at least 1 of the retrieved samples. Patients with non-diagnostic F-R-EBUS had a multidisciplinary evaluation and were referred either for another invasive procedure (i.e. trans-thoracic needle aspiration, surgical biopsy), or a clinical and radiological follow-up according to Fleischner Society guidelines.
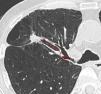
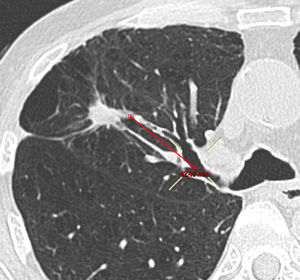
Imaging StudiesA radiologist specialized in thoracic imaging reviewed the last available chest CT scan performed before bronchoscopy and reported PPL type (solid, part solid, or pure ground glass opacities), size (greater transverse axis) and distance from the hilum. This distance was estimated on oblique reformatted images by measuring the distance between the internal margin of the PPL and the proximal origin of the corresponding segmental bronchus. The presence of a CT bronchus sign was evaluated using both axial and reformatted images.17
Statistical AnalysisThe data were tested for normal distribution using the Kolmogorov-Smirnov test and were analyzed in relation to both VY and DY using x2 and/or t-tests. Unless otherwise stated, results are presented as mean (range) or as number of cases (%). A P<.05 was considered statistically significant. The main operator learning curve was created by dividing all consecutive cases into four sub-groups of equal size. All statistical calculations were performed using R-statistics software.
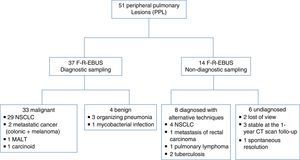
ResultsForty-eight consecutive patients [mean age (range); 66.1 (39–89) years] underwent bronchoscopy for study of PPL, and 51 PPLs were sampled with F-R-EBUS. Thirty-five PPLs (68.6%) were located in the upper, 2 (3.9%) in the middle, and 14 (27.5%) in the lower lobes. The mean lesion size was 25.8mm (range 8–67mm), 38 (74.5%) lesions were solid, 4 (7.8%) were pure ground glass, and 9 (17.7%) were part solid. Forty-two PPLs were visualized by F-R-EBUS resulting in a VY of 82.3%. F-R-EBUS sampling was diagnostic for 37 PPLs (DY of 72.5%) with 33 (89.2%) PPLs found to be malignant. The diagnostic contributions of the different bronchoscopic tools are summarized in Table 1. Fig. 1 presents a flow-chart diagram of the diagnostic procedures performed and the final diagnosis of PPL. No false-positive results were observed for F-R-EBUS biopsies, as confirmed by surgical sampling (26 nodules) and/or 1-year clinical and radiological follow-up. After exclusion of the six inconclusively diagnosed cases, the prevalence of lung cancer was 66.6% (n=34, 33 NSCLC and 1 carcinoid) and the sensitivity of F-R-EBUS for lung malignancies was 88.2% (n=30).
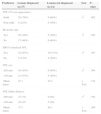
F-R-EBUS Diagnostic Contribution in 51 Cases.
| Method | Frequency of use No. of cases (%) | Establishment of diagnosis No. of cases (%) |
|---|---|---|
| Bronchoalveolar lavage | 25 (49%) | 8 (32%) |
| Bronchial washing | 51 (100%) | 19 (37%) |
| Brushing | 42 (82%) | 20 (48%) |
| Fine needle aspiration | 19 (37%) | 13 (68%) |
| Transbronchial biopsy | 42 (82%) | 21 (50%) |
DY and VY depended on the size of the lesion, being 64.0% and 80.0%, respectively, for PPL≤20mm (n=25), and 80.8% and 84.6%, respectively, for PPL>20mm (n=26). Regarding other radiologic nodule characteristics including PPL-pleura distance, the VY significantly improved (P=.021) only when the distance from the hilum (Fig. 2) was <50mm, while no radiologic predictor was identified for DY (Tables 2 and 3).
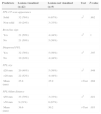
Predictors of Diagnostic Yield (DY).
| Predictors | Lesions diagnosed (n=37) | Lesions not diagnosed (n=14) | Test | P-value |
|---|---|---|---|---|
| PPL CT scan appearance | ||||
| Solid | 29 (78%) | 9 (64%) | x2 | .502 |
| Non-solid | 8 (22%) | 5 (36%) | ||
| Bronchus sign | ||||
| Yes | 20 (54%) | 5 (36%) | x2 | .392 |
| No | 17 (46%) | 9 (64%) | ||
| EBUS visualized PPL | ||||
| Yes | 32 (87%) | 10 (71%) | x2 | .397 |
| No | 5 (13%) | 4 (29%) | ||
| PPL size | ||||
| ≤20mm | 16 (43%) | 9 (64%) | x2 | .304 |
| >20mm | 21 (57%) | 5 (36%) | ||
| Mean (mm) | 27.1 | 22.3 | t-Test | .312 |
| PPL-hilum distance | ||||
| ≤50mm | 27 (73) | 9 (64) | x2 | .792 |
| >50mm | 10 (27) | 5 (36) | ||
| Mean (mm) | 37.1 | 44.1 | t-Test | .269 |
PPL, peripheral pulmonary lesion.
Predictors of Visualization Yield (VY).
| Predictors | Lesions visualized (n=42) | Lesions not visualized (n=9) | Test | P-value |
|---|---|---|---|---|
| PPL CT scan appearance | ||||
| Solid | 32 (76%) | 6 (67%) | x2 | .862 |
| Non-solid | 10 (24%) | 3 (33%) | ||
| Bronchus sign | ||||
| Yes | 21 (50%) | 4 (44%) | x2 | 1 |
| No | 21 (50%) | 5 (56%) | ||
| Diagnosed PPL | ||||
| Yes | 32 (76%) | 5 (56%) | x2 | .397 |
| No | 10 (24%) | 4 (44%) | ||
| PPL size | ||||
| ≤20mm | 20 (48%) | 5 (56%) | x2 | .948 |
| >20mm | 22 (52%) | 4 (44%) | ||
| Mean (mm) | 25.8 | 25.9 | t-Test | .988 |
| PPL-hilum distance | ||||
| ≤50mm | 33 (79%) | 3 (33%) | x2 | .021 |
| >50mm | 9 (21%) | 6 (67%) | ||
| Mean (mm) | 36.6 | 50.2 | t-Test | .053 |
PPL, peripheral pulmonary lesion.
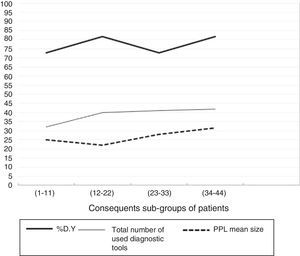
As previously mentioned, bronchoscopic procedures were performed by two experienced bronchoscopists with no previous specific experience with F-R-EBUS. One operator performed 44 interventions (86%) and the other 7 (14%). The learning curve (Fig. 3) of the main operator resulted in a DY of 72.7% (n=8) for the first subgroup, 81.8% (n=9) for the second, 72.7% (n=8) for the third, and 81.8% (n=9) for the last. The total number of diagnostic tools used and the mean PPL size were similar for all four groups of consecutive patients (32, 40, 41 and 42 diagnostic tools, and mean PPL size of 25.0mm, 22.1mm, 28.1mm and 31.5mm, respectively). Concerning the safety of F-R-EBUS, there was a single episode of significant hemorrhage which required interruption of the procedure without further complications.
DiscussionIn this study, we evaluated the diagnostic yield and safety of R-EBUS combined with fluoroscopy guidance, without guide sheath, in the study of PPL. We obtained a global diagnostic yield (DY) of 72.5% (64% for PPL≤20mm and 81% for PPL>20mm). The technique was simple to learn and its safety profile was similar to other EBUS-based techniques (no pneumothorax, only one significant hemorrhage).18
The aforementioned results are in the same range as those reported for all guided-bronchoscopy methods in the meta-analysis of Wang Memoli et al.19 (pooled DY of 70%, weighted DY of 60.9% for PLL≤20mm and 82.5% for PPL>20mm). These guided methods have been shown to be superior to traditional bronchoscopy without fluoroscopy.20,21 This good DY is probably related to the association of two diagnostic techniques, one permitting navigation toward the lesion, and the other permitting lesion visualization. The relevance of this association was highlighted in the clinical trial of Eberhardt et al.10 in which the authors used electromagnetic navigation bronchoscopy (EMN) as a “GPS” to guide the bronchoscopist within the bronchial tree and the EBUS to confirm the site of the lesion. They provided the highest DY (88%) reported so far in the literature, and demonstrated that the combination of the two methods is more efficient than either one alone. Although the electromagnetic field and the ultrasound system are safe, non-irradiating techniques, their combination is complex, and particularly heavy on resources; a specific chest CT-scan compatible with the EMN parameters should be repeated in most cases, two operators are required, and deep sedation or general anesthesia are used to fully immobilize the patient. Thus, the procedure is closer to an operation than a simple diagnostic intervention, its duration is longer and its cumulative cost is significant, making it difficult to use in daily practice.
Virtual bronchoscopic navigation (VBN) is another method by which images of the bronchial path to the lesion are generated and used as a guide to navigate the bronchoscope. Ishida et al. also demonstrated the importance of associating a guidance method with a target confirmation method by comparing the traditional EBUS-GS technique to the combination EBUS-GS+VBN11 approach, showing a significant improvement of DY in the combination arm.
Compared to the aforementioned highly effective combined techniques, the strength of our procedure lies in its simplicity, speed, and limited cost. Since both radioscopy and ultrasound processors are available in most endoscopic settings (the latter frequently being compatible with mediastinal-EBUS/EUS or urological/gastroenterological ultrasound systems), the purchase of a small, reusable (50 acts) EBUS probe provides a low-cost system of guidance and real-time PPL visualization (€3300 for Olympus products). The examination is rapid, adding 1–5min to the procedure, and it is performed under light sedation in an outpatient setting. The DY is slightly lower than reported by Eberhardt or Ishida et al., but the simplicity of the procedure could justify its first-line use, reserving more complex methods for cases of negative and doubtful results.
The advantage of using fluoroscopic guidance in addition to the R-EBUS seems to be a greater DY for PPL<20mm: Yoshikawa et al. reported a DY of 76% (n=28) for PPL≤20mm, in contrast to a DY of 30% (n=11) without the support of fluoroscopy.22
The guide sheath (GS) is neither a guidance method nor a real time lesion visualization method. Its utility lies in the repeatability of samplings at the same point of interest, and it becomes especially useful in patients with complex bronchial pathways. However, it requires additional manipulations to match the length of the tools, and is more expensive (€204–€276 for a single-use guide sheath). Our study confirms the efficiency of the simple association of radial probe endobronchial ultrasound and fluoroscopy without the guide sheath. Thus, the latter appears to be more an aid for repeated sampling than a tool for improving DY.
As shown by the learning curves of the technique, F-R-EBUS was associated with an immediate high diagnostic yield. DY remained high throughout the study and was not affected by PLL size or the number of diagnostic interventions. The aforementioned findings reflect the simplicity of the method, which, as shown by our experience, required no prior specific training and was directly applicable. As previously shown by Chao et al.23 FNA had the highest DY compared to the other diagnostic tools, and was not associated with more complications. This high DY is probably associated with the fact that FNA permits sampling of both endobronchial and extrabronchial lesions making this diagnostic tool a particularly useful technique in the clinical setting.
Predictors of DY inherent to R-EBUS have been widely investigated. In the meta-analysis of Steinfort et al. VY affected sensitivity irrespective of the guidance method.18 Radiological predictors for VY were analyzed by two retrospective studies that found a significant effect in both distance from the hilum24 and PPL size.16,24 In this study, only the distance from the hilum tended to correlate with the visualization yield. This lack of statistical significance probably reflects the fact that our study had a smaller patient population than previous investigations.16,24
Some of the innate limitations of a retrospective analysis, such as possible selection bias favoring patients at high-risk for malignancy and bronchoscopically accessible PPL, cannot be excluded in this study. Nevertheless, the diagnostic yield reported here lie on the linear regression line between the prevalence of lung malignancy and R-EBUS sensitivity performed by Steinfort et al.18 Another limitation, inherent to most trials studying the diagnosis of PPL, concerns the establishment of diagnosis and the histocytological criteria used for malignancy. In our study, the combination of histopathological criteria and clinical follow-up in uncertain cases resulted in no false-positive cases. Finally, a new method based on oblique reformatted images was used to estimate the distance between the hilum and the PPL (Fig. 2). The study of Tay et al. introduced the Pythagoras formula for calculating the distance of the PPL from the hilum on a 3D perspective.24 However, with this method the real bronchial pathway distance is not taken into account. In this study, oblique reformatted images permitted the calculation of the distance between the most internal PPL margin and the origin of the segmental bronchus in question, giving a more accurate estimation of the bronchial pathway to be followed during the bronchoscopic intervention.
In conclusion, R-EBUS combined with fluoroscopic guidance is a safe, rapid and simple technique that could be readily used in the evaluation of PPL. The diagnostic yield of this technique was comparable to other guided-bronchoscopy methods19 and was high for PPLs both larger and smaller than 20mm. These characteristics, combined with its moderate cost, could make it a first-line procedure in the diagnostic intervention sequence of PPL.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Casutt A, Prella M, Beigelman-Aubry C, Fitting J-W, Nicod L, Koutsokera A, et al. Ecografía endobronquial radial guiada por fluoroscopia sin vaina guía para lesiones pulmonares periféricas: una relación segura y eficiente. Arch Bronconeumol. 2015;51:338–343.