Several errors have been detected in Tables 1 and 5, in Fig. 1, and in the text of the article “Guidelines for the medical treatment of idiopathic pulmonary fibrosis” [Arch Bronconeumol. 2017;53:263–9]”.
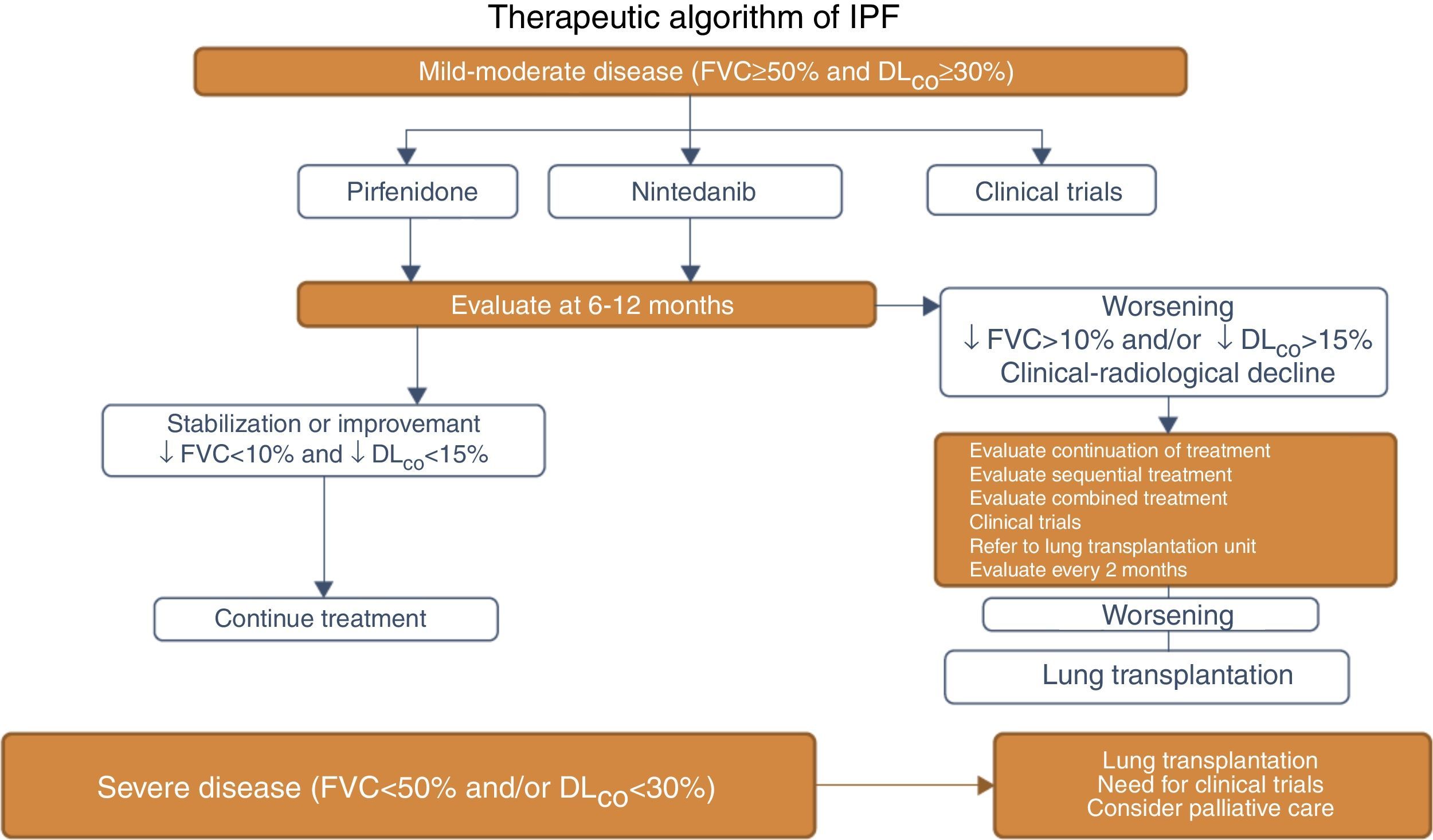
CAPACITY-1 and -2 and ASCEND Clinical Trials: Methodology and Clinical Features of IPF Patients Included.
| CAPACITY-1 and -2 (n=779) | ASCEND (n=555) | |
|---|---|---|
| Age (years) | 40–80 | 40–80 |
| FVC (inclusion criterion) | ≥50%–90% | 50–90% |
| DLCO (inclusion criterion) | ≥35%–90% | ≥30%–90% |
| Phase | III | III |
| Study duration (weeks) | 72 | 52 |
| UIP on HRCT | 92% | 95.7% |
| Initial FVC (mean) | 74.1% | 67.8% |
| Initial DLCO (mean) | 47% | 43.7% |
DLCO: carbon monoxide diffusing capacity; FVC: forced vital capacity; HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia.
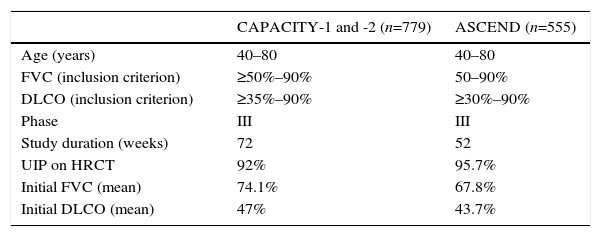
TOMORROW and INPULSIS Clinical Trials: Methodology and Clinical Features of IPF Patients Included.
| TOMORROW (n=422, 85 at doses of 150mg/12h) | INPULSIS-1 and -2 (n=1066) | |
|---|---|---|
| Age (years) | >40 | >40 |
| FVC (inclusion criterion) | ≥50% | ≥50% |
| DLCO (inclusion criterion) | 30%–79% | 30%–79% |
| Phase | II | III |
| Study duration (weeks) | 52 | 52 |
| UIP on HRCT | 38.8% | 76% |
| Initial FVC (mean) | 78.1% | 79.5% |
| Initial DLCO (mean) | – | 47% |
DLCO: carbon monoxide diffusing capacity; FVC: forced vital capacity; HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia.
The following tables are the correct versions:
The following text is the correct version:
- -
Page 3, section “Nintedanib (Ofev®)”, line 47. Where it says: “In the INPULSIS studies, patients with cardiovascular risk were excluded, so safety in this population could not be determined (Table 3). Table 4 shows nintedanib drug interactions.”, it should say: “Table 4 shows nintedanib drug interactions.”
- -
Page 5, section “Antifibrotics in Clinical Trials”, line 23. Where it says “Other combinations of pirfenidone or nintedanib with other new antifibrotics, initiated before either drug received marketing approval, are also ongoing (www.clinicaltrials.gov), including pirfenidone±lebrikizumab (RIFF study), pirfenidone or nintedanib±PRM-151 (PROME-DIOR study), pirfenidone±vismodegib (GB29764 study), pirfenidone±sildenafil.”, it should say: “Other combinations of pirfenidone or nintedanib with other new antifibrotics, initiated before either drug received marketing approval, are also ongoing (www.clinicaltrials.gov), including pirfenidone±lebrikizumab (RIFF study), pirfenidone or nintedanib±PRM-151 (PROME-DIOR study), pirfenidone±vismodegib (GB29764 study), pirfenidone±sildenafil, nintedanib+/−sildenafil (INSTAGE study) and other clinical trials in progress such as NCTO2606877 (nintedanib+pirfenidone and the INMARK study.”
- -
Page 5, the section title “Pulmonary Emphysema” should be replaced by: “Combined pulmonary fibrosis and emphysema (CPFE)”.
- -
Page 5, section “Pulmonary Emphysema”, line 1. Where it says: “No effective treatment is currently available for combined pulmonary fibrosis and emphysema (CPFE) syndrome,35,36 so it seems logical to base therapeutic decisions on the separate recommendations for emphysema and IPF.”, it should say: “No effective treatment is currently available for pulmonary syndrome,35,36 so it seems logical to base therapeutic decisions on the separate recommendations for emphysema and IPF.”
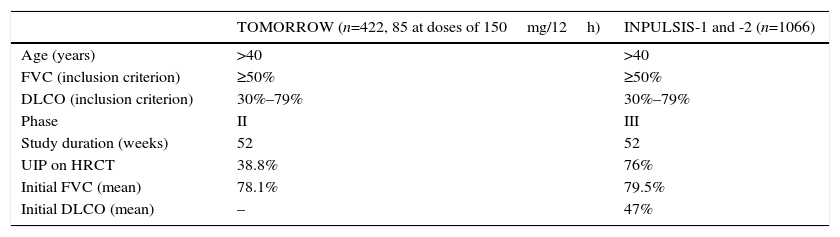
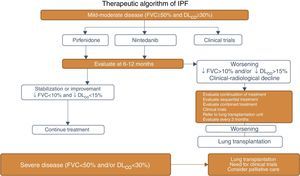
The following Fig. 1 is the correct version:
Please cite this article as: Xaubet A, Molina-Molina M, Acosta O, Bollo E, Castillo D, Fernández-Fabrellas E, et al. Fe de errores de «Normativa sobre el tratamiento farmacológico de la fibrosis pulmonar idiopática» [Arch Bronconeumol. 53 (2017) 263-9]. Arch Bronconeumol. 2017;53:657–658.