Inhaled medication is the first-line treatment of diseases such as asthma or chronic obstructive pulmonary disease. Its effectiveness is related to the amount of drug deposited beyond the oropharyngeal region, the place where the deposit occurs and its distribution (uniform or not). It is also important to consider the size of the inhaled particles, the breathing conditions, the geometry of the airways, and the mucociliary clearance mechanisms.
Currently, mathematical models are being applied to describe the deposition of inhaled drugs based on the size of the particles, the inspiratory flow, and the anatomical distribution of the bronchial tree. The deposition of particles in the small airways gets maximum attention from pharmaceutical companies and is of great interest as it is related with a better control in patients receiving these drugs.
La medicación inhalada constituye el tratamiento de primera línea de enfermedades como el asma o la enfermedad pulmonar obstructiva crónica. Su efectividad está en relación con la cantidad de fármaco que logre depositarse más allá de la región orofaríngea, con el lugar en que se produzca el depósito y con la distribución uniforme o no del mismo. Otros factores trascendentes son el tamaño de las partículas inhaladas, las condiciones de respiración, la geometría de las vías aéreas y los mecanismos de aclaramiento mucociliar.
Actualmente se están aplicando modelos matemáticos que permiten describir el depósito de fármacos inhalados a partir del tamaño de las moléculas, el flujo inspiratorio y la distribución anatómica del árbol bronquial. El depósito de partículas en las vías aéreas pequeñas recibe la máxima atención de las empresas farmacéuticas y es del máximo interés para poder controlar mejor a los pacientes que reciben estos fármacos.
The air that we breathe contains more than just nitrogen and oxygen. In addition, there are thought to be small concentrations of other gases (ozone, hydrogen, krypton, argon) and a variable quantity of water vapor depending on the environment in which we find ourselves. We do not know if this is all, although we fear that it is not. What we call “air” also contains enormous quantities of suspended particles, solid as well as liquid, organic and inorganic, bacteria, viruses, antigens, particles that can be more elemental, volatile or solid, simple or combined. All these elements make up the exterior environment where we breathe and live.
Our lungs are tremendous sponges of blood that also act as enormous filters that purify the air we breathe. By the time the oxygen and nitrogen molecules are deposited in the alveoli, we could say that they are practically free of the majority of these “contaminants” and that these local and general defense systems are quite effective during many, many years. This, of course, is true as long as we have taken care not to deliberately deteriorate our lungs by smoking.
The respiratory tract is especially designed, both anatomically and functionally, so that air can reach the most distal areas of the lungs in the cleanest possible condition. Nasal hairs, nasal turbinates, vocal chords, the cilia of the bronchial epithelium, the sneeze and cough reflexes, etc., all contribute to this filtering process. And, on most occasions it is properly done. But human beings are full of paradoxes: an efficient system, designed to avoid certain particles from penetrating into the lungs, is at the same time used to intentionally deposit drugs in the airways and even for these to reach the alveoli in the best possible condition. It is thus necessary to get around the defense systems by evading reflex arcs, mucus layers, ciliary movements, etc., so that, with the inspiratory flow, the molecules that can improve diseases are deposited in the lungs. A system that evolved over time in order to filter and clean the air should be dodged in order to deposit other substances that we deliberately want to reach the inside of the organism. Without a doubt, an understanding of anatomy, airway function and the laws of physics that govern flow dynamics, size, shape, and the number of inhaled particles will contribute to the development of this area of expertise. This area is so close to our specialty and has generated great interest, especially for the development of new methods to administer medication.
Factors That Affect the Deposition of Aerosolized DrugsParticle Size and ShapeThe size and shape of particles are primordial factors that condition their deposition in the lungs. The size is defined by what is called the mass median aerodynamic diameter (MMAD) or diameter of a particle of mass equal to the average particle diameter of a population, meaning the diameter of a particle in which 50% of the aerosol mass is greater and the other 50% is smaller.1 Depending on their size and shape, the particles can be deposited by means of four mechanisms:
- •
Impaction. This is the physical phenomenon by which the particles of an aerosol tend to continue on a trajectory when they travel through the airway, instead of conforming to the curves of the respiratory tract.2 Particles with enough momentum (product of the mass and velocity) are affected by centrifugal force at the points where the airflow suddenly changes direction, colliding with the airway wall. This mainly happens in the first 10 bronchial generations, where the air speed is high and the flow is turbulent.3 This phenomenon mainly affects particles larger than 10μm, which are mostly retained in the oropharyngeal region, especially if the drug is administered by dry powder inhalers (DPI) or metered-dose inhalers (MDI).4
- •
Interception. This is mainly the case of fibers, which, due to their elongated shape, are deposited as soon as they contact the airway wall.
- •
Sedimentation. This is the physical phenomenon by which particles with sufficient mass are deposited due to the force of gravity when they remain in the airway for a sufficient length of time. This predominates in the last 5 bronchial generations, where the air speed is slow and the residence time is therefore longer.3
- •
Suspension. This is the phenomenon by which the particles of an aerosol move erratically from one place to another in the airways. This happens as a consequence of the Brownian diffusion of particles with an MMAD smaller than 0.5μm when they reach the alveolar spaces, where the air speed is practically zero. These particles are generally not deposited and they are expelled once again upon exhalation.
The particles of aerosolized drugs usually have a uniform shape and are symmetrical on several planes. They rarely are smaller than 1μm, and therefore the predominating mechanisms are impaction and sedimentation.5
It can generally be considered that particles with an MMAD higher than 10μm are deposited in the oropharynx, those measuring between 5 and 10μm in the central airways and those from 0.5 to 5μm in the small airways and alveoli. Therefore, for topical respiratory treatment it is best to use particles with an MMAD between 0.5 and 5μm. This is what is known as the breathable fraction of an aerosol.6
Airflow VelocityBecause particles are transported through the airway by an air current, their trajectories are affected by its characteristics. The air flow in the lungs is determined by the tidal volume and respiratory rate. Sbirlea-Apiou et al.7 demonstrated that in the first four generations of the airway, the deposition increases for any size particle as the inspiratory flow increases. However, the opposite is true in the last generations of the airway, where the deposition of particles is inversely proportional to this flow. This is due to that fact that the increased inspiratory flow reduces the residence time of the particles in the airway, therefore the effects of the severity and of the Brownian movement will be quite lower. Obviously, a minimal inspiratory flow is necessary to drag the particles toward the interior of the bronchial tree.
Airway GeometryThe probabilities of particle deposition by impaction increase when the particles themselves are larger, the inspiratory airflow is greater, the angle separating the two branches is wider and the airway is narrower.8
In pathologies such as chronic bronchitis or asthma, which may alter the lung architecture with the appearance of bronchoconstriction, inflammation or secretion accumulation, the deposition of aerosolized drugs is modified. The smaller caliber of the airway increases air speed, producing turbulence in places where the flow is usually laminar. The airway obstruction also means that the air tends to be displaced toward unobstructed areas, and therefore the drug will also tend to be deposited mostly in healthy areas of the lung.9
Degree of HumidityThe particles of aerosolized drugs can be hygroscopic to a greater or lesser extent. Hygroscopicity is the property of some substances to absorb and exhale humidity depending on the setting in which they are found. This means that they can get larger or smaller in size upon entering into the airway, with the consequent modification in the deposition pattern compared to what was initially expected. The diameter that a particle reaches after hygroscopic growth depends on its initial diameter, the intrinsic properties of the particle, and the environmental conditions in the airways. The mole fraction of water vapor contained in the airway has been demonstrated to be an important factor related with the increase in the MMAD of the aerosol particles.10 In general, it is considered that hygroscopic growth does not have much of an effect in particles with MMAD less than 0.1μm; meanwhile it is very intense in particles with MMAD larger than 0.5μm.11
The hygroscopicity of molecules can be used to try to favor the deposition of inhaled drugs. Studies have been developed in which an aerosol was administered with a submicrometric or nanometric MMAD in order to reduce extrathoracic loss, taking advantage of later growth due to hygroscopicity, which enabled the particles to be retained within the lungs.12,13
Mechanisms for Mucociliary ClearanceOnce deposited in the airways, the particles can be carried by the mucociliary system, degraded or absorbed into the systemic circulation or the lymph ducts.9 The first of these mechanisms is found in the conducting airways (from the trachea to the terminal bronchioles), which have ciliated epithelium that are covered by two layers of mucus: a low-viscosity periciliary layer, or sol, and a thicker layer that covers the former, or gel. This biphasic layer of mucus protects the epithelium from dehydration, helping to humidify the air and providing a protective barrier by trapping inhaled particles.14 The insoluble particles are trapped by the gel and they are moved toward the pharyngolaryngeal region by the movements of the ciliated epithelium, where it is either coughed up or swallowed.15,16 The clearance speed depends on the number of ciliated cells and the cilia beat frequency, and it may be affected by factors that influence the function of the cilia or the quantity or quality of the mucus. In cystic fibrosis (CF), for example, very thick mucus is produced that does not correctly move along with the cilia. This is due to a mutation in the gene that codes the CFTR receptor, which regulates the passage of the chloride ion through the surface of the epithelial cells.17
The soluble particles are eliminated by absorptive mechanisms. The liposoluble molecules cross the respiratory epithelium by passive transport; the hydrosoluble molecules can cross the epithelial barrier either through the intercellular spaces or by active transport (by mechanisms of endocytosis and exocytosis).18 Once in the submucosal region, the particles can enter the systemic circulation, bronchial circulation or lymphatic system.9 The particles that are deposited in the alveoli can be devoured and eliminated by the alveolar macrophages if they are insoluble particles (non-absorptive mechanism)19; if they are soluble, they can be absorbed into the systemic circulation.20
Optimal Locations for Drug Deposition in the Treatment of Some Respiratory DiseasesThe administration of inhaled drugs presents a series of advantages over systemic administration, making it preferable for the treatment of local diseases. High doses of a drug may be administered and rapidly absorbed through the epithelium of the airways, meaning that the inhaled administration enables a drug to act quickly, while minimizing systemic side effects. If, however, an aerosolized drug is deposited at a suboptimal dose or in a region of the lung that is not affected by the pathology to be treated, the effectiveness of the treatment will be compromised.21 The receptors for β2 adrenergic agonists (salbutamol, terbutaline) and M3 muscarinic receptor antagonists (ipratropium bromide) are not uniformly distributed throughout the lung. More than 90% of the β receptors are located in the alveolar walls, and specifically there is a high density of β2 receptors located in the epithelium of the airway between the main bronchi and the terminal bronchioles.22 There is a high density of M3 receptors in the submucosal glands and lung lymph nodes, while there is a lower proportion in the smooth muscle of the airways, in the nerves that innervate the bronchi and in the alveolar wall.23 The location of these receptors in the lung suggests that ipratropium bromide should be deposited in the conducting airways in order to reach a greater effectiveness; meanwhile, salbutamol should be deposited more peripherally (in the middle and small airways) to produce an adequate therapeutic effect. In the case of inhaled corticoids, the treatment seems to be more beneficial when more of the drug is dispersed throughout the lungs, as inflammatory cells such as eosinophils, lymphocytes, and macrophages are present throughout the respiratory tract and alveoli in asthma patients.24,25 The optimal location for the deposition of aerosolized antibiotics depends on the disease to be treated. In the case of CF, there is chronic colonization by Pseudomonas aeruginosa, which tends to grow in the lumen of the airways, with a limited invasion of the pulmonary parenchyma. The infection starts in the bronchioles and moves toward more proximal airways26; therefore, the ideal place for deposition of inhaled antibiotics would be throughout all the conducting airways. Mucus accumulations in certain areas can impede the antibiotics being deposited in regions behind the obstruction, which are presumably the most infected areas, and the effectiveness of the treatment is therefore compromised.9,27
Devices for the Administration of Inhaled DrugsThe treatment of respiratory infections with inhaled steam has been traditionally done for as long as anyone can remember. In 1828, Schneider and Waltz developed an atomizer for mineral water sprays, but it was also used as an inhaler. The first portable inhaler was created in 1856 by Sales-Giron, a physician at a spa. It consisted of a manual liquid atomizer that enabled patients to administer inhaled balsamic infusions at home. The discovery of adrenalin in 1901 by Takamine and Aldrich, and its inhaled administration for the first time in 1929,28 initiated the search for and administration of new inhaled drugs, leading to improvements in the devices for their administration.29
The devices currently used for the administration of inhaled drugs can be divided into three types: nebulizers, metered-dose inhalers, and dry powder inhalers.
NebulizersThere are basically two types of nebulizers: jet and ultrasonic. Jet nebulizers are based on the Bernouilli effect, according to which a compressed gas that passes through a narrow orifice creates a low-pressure area upon exiting. If at this low-pressure point we connect a tube that has a thin layer of liquid, the low pressure will cause this liquid to be aspirated in small droplets. Ultrasonic nebulizers use piezoelectric crystals that vibrate at a high frequency within the nebulizing chamber, transmitting the vibratory energy to the liquid that is in contact with it, converting said liquid into an aerosol.30 Jet nebulizers can generally aerosolize most drug solutions, and ultrasonic nebulizers may not be effective if viscous suspensions or solutions are used.31
Nebulizers can administer high doses of medication in patients who are not able to coordinate or cooperate and they are able to administer several substances mixed together in one same solution. The minimal inspiratory flow needed for the aerosol produced by a nebulizer to reach the lungs is 6–8l/min.2 However, there are high amounts of drug lost as much of the medication is retained in the nebulizer dead-space, or it is lost in the room air during expiration. It has been estimated that only 10% of the dose that is initially placed in the nebulizer will be effectively deposited in the lungs.32 The large droplets are deposited in the oropharynx, while the droplets are too small to penetrate in the lungs and are once again expelled during expiration.
Pulmonary deposition may be increased by modifying the patient's way of inhaling. Most patients inhale by using circulating volume. If the patient takes a deep breath and holds it in, the quantity of medication retained in the lungs may increase 14%–17%.8 Probably the most practical way to modify the deposition pattern is to reduce the size of the droplets that are generated. This can be done with ultrasonic nebulizers by making the piezoelectric crystal vibrate at a greater frequency; doing so with jet nebulizers can increase the compressed gas flow.33
Metered-dose InhalersMetered dose inhalers (MDI) are devices used to administer aerosolized drugs that emit a fixed dose of medication with each pulse. They have a metallic chamber containing a suspension or solution of the drug with a liquid propellant that, at room temperature and atmospheric pressure, turns to its gaseous phase. A key piece in this system is the dosage valve, which releases at each pulse a controlled, reproducible dose of medication. The drug is released at a high speed (at more than 30m/s through the mouthpiece) and in the form of particles with an MMAD of between 2 and 4μm.34 MDI have a series of advantages, such as their small size (making them easy to handle), the exactness of the dosage, the possibility to fit them to spacer chambers, the fact that they do not require high flows to be inhaled and their low cost in general. Their main drawbacks are the difficulty inherent in synchronizing activation–inhalation and the low dose that reaches the lungs, which has been estimated at approximately 10%–20% of the dose emitted.35,36 The high release speed and the large size of the particles generated mean that more than half of these impacts in the oropharyngeal region.36 Another drawback of MDI is the possible variation in the dose released at each pulse if the device is not correctly shaken.34
In the past, the propellant used was chlorofluorocarbons (CFC), but due to their harmful effects on the ozone layer, they have been banned by the United Nations. The substitute currently used in MDI are hydrofluoroalkanes (HFA).37 HFA transform into their gaseous state at a higher temperature than CFC,38 reducing the cold freon effect, which is the interruption of breathing when the particles impact against the back wall of the oropharynx. There are currently on the market MDI-HFA with salbutamol, fluticasone, beclomethasone, anticholinergics, and the combination of salmeterol–fluticasone.34 The development of MDI with HFA has also been able to reduce the size of the aerosol droplets and, therefore, improve the lung deposition of the drug. In the case of beclomethasone–HFA, with an MMAD of 1.1μm, it has been demonstrated that up to 56% of the initial dose is deposited.39–41
The optimal conditions for the inhalation of an aerosol using an MDI are to start breathing from functional residual capacity, activating at that moment the inhaler, inhale using an inspiratory flow less than 60l/min and follow the inspiration with 10s of apnea.42 This method increases deposition by sedimentation in the more peripheral areas of the airway. The minimal inspiratory flow necessary for its use is approximately 20l/min.2
One way to avoid the lack of coordination between the patient and the device are to use inhalation chambers that fit on the mouthpiece of the MDI. The aerosol goes into the chamber and the particles that are too big impact against its wall and are retained there, while the smaller particles remain in suspension within the chamber until they are inhaled by the patient. In addition, the space that the chamber provides between the MDI and the mouth of the patient allows the aerosol to lose speed, reducing impaction against the oropharynx. In this manner, local adverse effects are reduced and the lung deposition of the drug is increased.43 It has been demonstrated that MDI used with inhalation chambers are as effective as nebulizers in the treatment of acute asthma attacks.44
Furthermore, with the aim of avoiding a lack of coordinated activation and inhalation, new MDI have been developed that automatically release the medication when the patient inhales, such as Autohaler® and Easybreath®; these devices have been shown to improve the lung deposition of drugs in patients for whom coordination is difficult.45 In addition, they require less inspiratory flow than conventional MDI, at around 18–30l/min, which makes them more adequate for patients with physical limitations, children and the elderly.34,46
Dry Powder InhalersDry powder inhalers (DPI) were designed with the aim to eliminate the inherent coordination difficulties of MDI. They administer individual doses of drugs in a powder form contained in capsules that should be broken open before their administration (unidose systems), or in blisters that move around in a device or have powder reservoirs (multidose systems).
Other advantages of DPI are that they do not require propellants for their administration, which makes them more respectful of the environment, and many of them have an indicator of the doses remaining. The main drawbacks are that patients perceive to a lesser degree the drug entering the airway, which may complicate treatment compliance, and its price is generally higher than that of MDI. DPI should be stored in a dry setting, as humidity favors the agglomeration of the powder that can obstruct the inhalation system.37
The dose that reaches the lungs is similar to MDI, and less than 20% of the initial dose actually reaches the lungs. It has been demonstrated that if the inhalation technique is correct, there is no difference between the administration of a drug by means of DPI or MDI.47 The use of low inspiratory flow, humidity, and changes in temperature are all factors that have been shown to worsen the lung deposition of medications with DPI.48
In the case of DPI, the “aerosol” is produced by the inspiratory effort of the patient.47 An inspiratory flow of at least 30l/min is necessary for the powder medication to become dispersed and reach the lungs, which may be difficult to be done in the elderly, children or patients with severe respiratory disorder.49 The air is directed toward the container with loose powder, which generally consists of particles that are too large to penetrate the airway due to the formation of powder agglomerations or to the presence of large-sized particles that transport the drug, such as lactose. The dispersion of the powder into particles that enter into the inhaled fraction is produced by the formation of turbulent airflows inside the powder container, which break the powder agglomerations up into smaller-sized particles and separate the transport particles from the drug.50 The particles that are generated have a final MMAD that ranges from 1 to 2μm.51,52 Every DPI has a different airflow resistance that determines the inspiratory effort necessary to disperse the powder. The greater the resistance of the device, the more difficult it is to generate the inspiratory effort, but at the same time the deposition of the drug in the lungs is greater.53,54
Methods for Studying the Lung Deposition of ParticlesThe first models of aerosol deposition in the lungs were based on very simple pulmonary morphologies. They used a small number of breathing conditions and a limited range of particle sizes, and were usually models confined to an area of the respiratory tract instead of models of the entire respiratory tract. In addition, they were limited to aerosols generated in industrial settings, like mining.
The first mathematic model of particle deposition was done in 1935 by Findeisen. This author, basing his study on the anatomical understanding of the age, divided the respiratory tract into only 9 generations, reaching the alveolar sacs and ducts. This model assumed a series of dimensions, flow speeds, transit times, and types of ramification for each generation, and formulas were established in order to calculate the particle deposition in each generation according to the 3 basic mechanisms of deposition: impaction, sedimentation, and diffusion. The main limitations of this model are that the airways above the trachea were not contemplated, and the anatomic simplicity of the lower airway model used. However, this pioneering model established the basic norms for the development of other later models.55
Another significant model was that by Landhal in 1950, which added two new compartments to the Findeisen model: the mouth and pharynx.56 Later, Beekmans presented a new model in 1965, in which the 3 basic deposition mechanisms were assumed, and an attempt was made at correcting the dimensions of the airway caused by its expansion during inspiration. In addition, it considered the role of the mix between tidal and residual volume in the three last generations of the airway. In this model, Beekmans established equal inspiratory and expiratory times, and after every phase he established a pause in which the deposition was produced by diffusion and sedimentation.57
A more anatomically detailed model of the airways was published by Davies: including 15 generations, it starting in the mouth and ending in the alveolar sacs. Nevertheless, this was not the basis used to develop a mathematical model calculating particle deposition.58 The most commonly used anatomical model was the Weibel model. In this model, the ways of bifurcation are indicated, designating the trachea as the first airway (order 0) and presuming that each airway leads to two branches (regular dichotomy). Weibel described a minimum of 23 bronchial generations up to the alveolar ducts.59
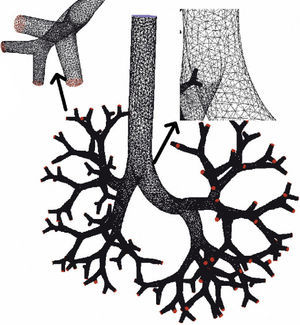
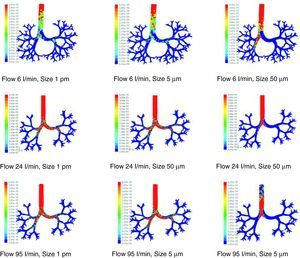
There are many other later theoretical studies about the behavior of flow in the airways.60–69 These studies have generally been focused on isolated sections of the lung, such as the trachea and the first generations of the airway or the alveolar ducts and sacs. Currently, the predominating studies are based on computational fluid dynamics (CFD). CFD is a technique that tries to use computers to simulate the movement of fluids and to resolve the mathematic equations governing their movement (Navier–Stokes equations). In this way, the behavior of a fluid and the particles that travel in it can be simulated. By means of CFD it is possible to develop a model of the airways, with any desired degree of detail, in order to simulate the behavior of air and the aerosol particles within it (Figs. 1 and 2).70 Thoracic computed tomography (CT) images of a patient may be used to develop a personalized model and to check the deposition of inhaled drugs in that patient.71
Results of the simulation with computational fluid dynamics (CFD) of particle behavior at different sizes (1pm, 5μm, and 50μm), which are dragged by flows of 6, 24, and 95l/min. The red areas indicate a high density of trapped particles. It can be observed that, as the size of the particle and flow increase, more particles tend to become trapped in more central regions of the airway due to impaction.
At the same time these mathematical models were arising, numerous experimental models about particle deposition were developed, including those by Drinker, Brown, Patterson, etc.,72–74 whose results generally did not differ from the mathematical models. These experimental studies generally calculated total aerosol deposition by measuring the quantity that entered and exited the respiratory tract.75
Nowadays, the most commonly used techniques for determining the distribution of inhaled drugs are gammagraphy and single photon emission computed tomography (SPECT) in 3 dimensions (3D), although other techniques, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), are gaining importance. These techniques are used in combination with drugs or radioactively marked molecules.76
The isotope usually used in gammagraphy and in 3D SPETC is technetium-99m (99mTc), which is associated with the drug being studied without being part of it. Gammagraphy provides lung images in 2 dimensions (2D), and it has frequently been used to compare the effectiveness of aerosol lung deposition using different inhalation devices, as well as the effects of different respiratory and lung disease parameters on the deposition. The distribution of the drug is generally studied according to areas of interest, meaning comparing the apical and basal regions, or central and peripheral distributions.76
SPECT can obtain accumulative 2D images of the thorax of the patient, which gives more precise images in order to evaluate drug deposition patterns in the lungs. However, this is not possible if there is no unabsorbable direct radioactive marker available for the drug being studied. It is useful, on the other hand, for the study of variables that are secondary to the inhalation of medication, such as pulmonary perfusion and ventilation, mucociliary clearance or pulmonary epithelial permeability.76
In the case of PET, the markers used are usually carbon, fluoride, nitrogen, and oxygen, which are atoms that make up any organic molecule, and it is therefore simpler to mark the drug being studied. The markers that are most commonly used are C11 and F18. The images obtained by PET can be divided into areas that are either more central or more peripheral and correlated with the degree of radioactivity detected and, therefore, with the dose of drug deposited in each region.76
DiscussionInhaled medication is the first line of treatment in disease such as asthma or COPD. Laboratories continuously study new inhalation devices that would provide better drug deposition in the lungs. In order for an aerosolized drug to be effective, an adequate quantity of it should be able to be deposited beyond the oropharyngeal region. The location of the deposition (central or peripheral airways) and the uniform or non-uniform distribution of the inhaled drug also play an important role in its effectiveness.
The effect of aerosol therapies depends on the dose deposited as well as its distribution in the lungs. If an aerosol is deposited at a suboptimal dose or in a region of the lung that is not affected by the pathology being treated, the efficacy of the treatment will be compromised.
Factors such as the size of the aerosol particles, breathing conditions, the geometry of the airways or mucociliary clearance mechanisms play a fundamental role in the lung deposition of aerosolized drugs.
These peculiarities of each individual make it necessary to have available in clinical practice some type method that would be able to personalize aerosolized therapies. One way to achieve more personalized treatment would be to create airway models that are exclusive for each patient using CFD techniques. Hospitals now have more powerful, high-resolution scanners and software that create three-dimensional reconstructions of the bronchial tree. These images would be an important data source for the construction of a model that would provide flow and particle deposition analysis using CFD techniques. The possibilities of CT for studying the airway are underestimated.
In this current phase of our knowledge and understanding, these mathematical models, calculated by applying CFD techniques with information from HRCT, should be compared and evaluated with the classic approach provided by gammagraphy images or PET. We believe that the application of these hypothetical models will enable drug deposition to be studied and new inhalation devices to be designed that contribute toward the improved health state of our patients. In any event, a good source of “inspiration” could be the way in which smoker patients inhale.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Fernández Tena A, Casan Clarà P. Depósito pulmonar de partículas inhaladas. Arch Bronconeumol. 2012;48:240–6.