Community-acquired pneumonia (CAP) is a prevalent disease among children and is frequently associated with both diagnostic and therapeutic uncertainties. Consensus has been reached between SEPAR, SENP and SEIP, and their conclusions are as follows:
Etiology depends mainly on age and other factors and no single analytical marker offers absolute diagnostic reliability.
In the event of clinical suspicion of pneumonia in a healthy child, chest X-ray is not necessary. Chest ultrasound is increasingly implemented as a follow-up method, and even as a diagnostic method.
The empirical antibiotic treatment of choice In typical forms of the disease is oral amoxicillin at a dose of 80 mg/kg/day for 7 days, while in atypical presentations in children older than 5 years, macrolides should be selected. In severe typical forms, the combination of 3rd generation cephalosporins and cloxacillin (or clindamycin or vancomycin) administered intravenously is recommended.
If pleural drainage is required, ultrasound-guided insertion of a small catheter is recommended. Intrapleural administration of fibrinolytics (urokinase) reduces hospital stay compared to simple pleural drainage.
In parapneumonic pleural effusion (PPE), antibiotic treatment combined with pleural drainage and fibrinolytics is associated with a similar hospital stay and complication rate as antibiotic treatment plus video-assisted thoracoscopy (VATS).
Systematic pneumococcal conjugate vaccination is recommended in children under 5 years of age, as it reduces the incidence of CAP and hospitalization for this disease.
La neumonía adquirida en la comunidad (NAC) es una enfermedad prevalente en la edad pediátrica y que ofrece frecuentemente dudas tanto diagnósticas como terapéuticas. Se ha realizado un consenso entre SEPAR, SENP y SEIP, con las siguientes conclusiones:
La etiología depende fundamentalmente de la edad y de otros factores, como estado inmunitario, presencia de enfermedad de base o estado vacunal y no existe un marcador analítico único con una absoluta fiabilidad diagnóstica.
Ante la sospecha clínica de neumonía, no es imprescindible la realización de una radiografía de tórax en los niños sanos. La ecografía torácica se va imponiendo como método de seguimiento, e incluso de diagnóstico.
El tratamiento antibiótico empírico de elección en las formas típicas es la amoxicilina oral a una dosis de 80 mg/kg/ día generalmente durante 7 días, mientras que en las atípicas en mayores de 5 años son los macrólidos. En las formas típicas graves se recomienda la combinación de cefalosporina de 3.a generación y cloxacilina (o clindamicina o vancomicina) por vía intravenosa.
En caso de requerir drenaje pleural, se recomienda la inserción ecoguiada de un catéter de pequeño tamaño. La administración intrapleural de fibrinolíticos (urocinasa) reduce la estancia hospitalaria en comparación con el drenaje pleural simple.
En el derrame pleural paraneumónico el tratamiento con antibioticoterapia junto con drenaje pleural y fibrinolíticos se asocia con una estancia hospitalaria y una tasa de complicaciones similar al tratamiento antibiótico más videotoracoscopia asistida.
Se recomienda la vacunación antineumocócica conjugada sistemática en menores de 5 años, ya que reduce la incidencia de NAC y de hospitalización por esta causa.
The definition of community-acquired pneumonia (CAP) is complex and varies widely among the different guidelines.1,2 Some are based solely on clinical criteria, while others also take into account radiographic findings or laboratory data.
CAP is defined as an acute infection of the lung parenchyma in a patient exposed to a microorganism outside the hospital setting. A standard criterion for CAP diagnosis is no history of hospital admission within 7–14 days prior to the onset of symptoms or onset of symptoms within 48 h of hospitalization.3
The current evidence on clinical presentation, diagnosis and treatment of CAP in children has been reviewed by members of the Pediatric Pulmonology area of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), the Spanish Society of Pediatric Pulmonology (SENP), and the Spanish Society of Pediatric Infectious Diseases (SEIP). Recommendations have been made according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system (Appendix B Annex). On the basis of the evaluation, the quality of evidence for each outcome is classified into 4 categories: high, moderate, low, or very low. In areas where scientific evidence is insufficient, recommendations agreed by consensus among the authors have been made.
Incidence and mortalityAccording to data from the World Health Organization, CAP accounted for 15% of deaths among children under 5 years of age in 2015, and more than 900,000 deaths among children of all ages worldwide.4 Pneumonia is the greatest single cause of infant mortality worldwide.1 In developed countries, however, this rate is virtually zero in children without previous comorbidities.
Regarding hospitalization for CAP, in the Etiology of Pneumonia in the Community (EPIC) study conducted in 2638 children in the US, a hospital admission rate of 15.7 per 10,000 was observed in children under 18 years of age, rising to 62.2/10,000 in children under 2 years of age.5 In European countries, the incidence of consultations in hospital emergency departments for CAP is 14.4/10,000 in children aged 0–16 years and 33.8/10,000 in children under 5 years.2 However, in recent years, since the introduction of pneumococcal vaccination, a significant reduction in hospitalization for this cause has been reported.6,7 A recent study8 conducted in Spain between 2001 and 2014 observed an annual reduction of 3.4% in the pneumonia admission rate in children under 2 years of age. In the same period, these and other authors describe a parallel increase in the frequency of empyema, which they attribute, among other causes, to an increase in the prevalence of different pneumococcal serotypes capable of invading the pleural space that were not included in the 7-valent pneumococcal conjugate vaccine (PCV7), primarily serotypes 19A, 1 and 7 F.9 This increase in the frequency of complications had already been observed in recent decades, even before the introduction of PCV7, so it is possible that other factors, such as the use of antibiotics, antimicrobial resistance, or the epidemiological trend itself, might also have played an important role.
Risk factorsThe development of pneumonia in children and its clinical severity are the result of a complex interaction between host and environmental factors.10 In healthy children, especially boys, the greatest risk is to be younger than 5 years of age and, in particular, younger than 2.8 In terms of underlying diseases, asthma is the most common in children hospitalized for CAP, followed by neurological diseases,8 which are associated with higher morbidity and mortality. Other risk factors include: bronchopulmonary dysplasia, congenital heart disease, prematurity, immune deficiency, overcrowding, exposure to environmental pollution, etc.11,12
It has been suggested that certain polymorphisms in genes involved in the innate or specific immune response may be associated with increased susceptibility to developing CAP, as can be seen from the results of a recent meta-analysis in which adult carriers of the Toll-like receptor 4 (TLR4) A299 G polymorphism seemed to present a higher risk. However, these conclusions are not applicable to children, because only 1 pediatric study was included.13
Because of the efficacy of vaccines against pneumococcus and Haemophilus influenzae (H. influenzae) type b, failure to vaccinate should be considered one of the most important preventable risk factors for the development of CAP in childhood.
Host-related factors associated with more clinically severe CAP include: comorbidities, age under 2 years, absence of breastfeeding or duration less than 4 months, malnutrition, and passive smoking.8,14
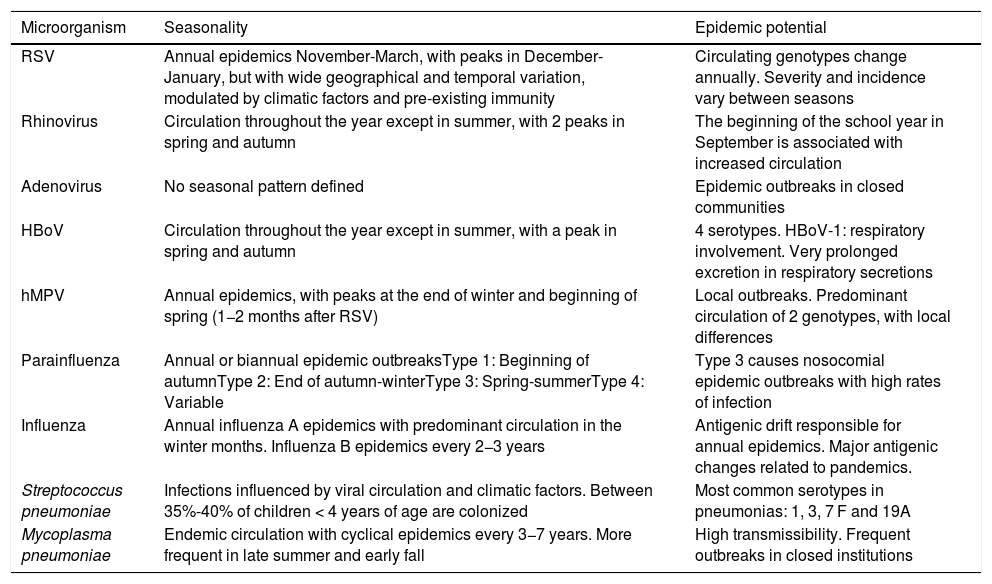
SeasonalityThe epidemiology of CAP is seasonal and closely related to the circulation of the major etiological agents (Table 1).15 In temperate countries, the highest incidence of CAP is recorded in the winter months, coinciding with the peak circulation of respiratory viruses, especially respiratory syncytial virus (RSV).16 It has been suggested that colonization by both pneumococcus and RSV triggers a synergistic effect that favors the pneumococcal infection and the pulmonary inflammatory response, increasing the incidence of CAP, with or without bacteremia.17 Moreover, co-infection with RSV appears to increase pneumococcal virulence and clinical severity.17,18
Seasonality of the main etiological agents of community-acquired pneumonia in children.
| Microorganism | Seasonality | Epidemic potential |
|---|---|---|
| RSV | Annual epidemics November-March, with peaks in December-January, but with wide geographical and temporal variation, modulated by climatic factors and pre-existing immunity | Circulating genotypes change annually. Severity and incidence vary between seasons |
| Rhinovirus | Circulation throughout the year except in summer, with 2 peaks in spring and autumn | The beginning of the school year in September is associated with increased circulation |
| Adenovirus | No seasonal pattern defined | Epidemic outbreaks in closed communities |
| HBoV | Circulation throughout the year except in summer, with a peak in spring and autumn | 4 serotypes. HBoV-1: respiratory involvement. Very prolonged excretion in respiratory secretions |
| hMPV | Annual epidemics, with peaks at the end of winter and beginning of spring (1−2 months after RSV) | Local outbreaks. Predominant circulation of 2 genotypes, with local differences |
| Parainfluenza | Annual or biannual epidemic outbreaksType 1: Beginning of autumnType 2: End of autumn-winterType 3: Spring-summerType 4: Variable | Type 3 causes nosocomial epidemic outbreaks with high rates of infection |
| Influenza | Annual influenza A epidemics with predominant circulation in the winter months. Influenza B epidemics every 2−3 years | Antigenic drift responsible for annual epidemics. Major antigenic changes related to pandemics. |
| Streptococcus pneumoniae | Infections influenced by viral circulation and climatic factors. Between 35%-40% of children < 4 years of age are colonized | Most common serotypes in pneumonias: 1, 3, 7 F and 19A |
| Mycoplasma pneumoniae | Endemic circulation with cyclical epidemics every 3−7 years. More frequent in late summer and early fall | High transmissibility. Frequent outbreaks in closed institutions |
HBoV: human bocavirus; hMPV: human metapneumovirus; RSV: respiratory syncytial virus.
Partially adapted from Clark et al.15.
The association of influenza virus with bacterial pneumonia, particularly when caused by pneumococcus and Staphylococcus aureus (S. aureus), is also an important factor.19
EtiologyGeneral aspectsThe etiological diagnosis of bacterial CAP in children is more complicated than in adults, as it is difficult to obtain samples from the lower airways and the routine use of invasive methods for these purposes in unfeasible.
VirusIn general, respiratory viruses play a key role in the etiology of CAP, whether they occur as a single infection or concomitantly with bacteria.20 The viral detection rate may reach 90%, especially in children under 2 years of age, while in school-age children it is somewhat lower (identified at a rate of 30%-60%).20–23
A Spanish study of children with CAP8 found a progressive increase in viral identification in recent years, mirrored by a decrease in Streptococcus pneumoniae (S. pneumoniae) isolation, possibly due to improved microbiological techniques, among other factors. Similarly, in the EPIC study, viruses were detected as the only etiological agent in 60% of pneumonia cases among children under 5 years of age in the US.5 However, while S. pneumoniae is significantly associated with severe CAP, identification of viruses is similar in mild and severe cases.24
RSV is the most frequently identified virus in CAP in children under 18–24 months of age, and rhinoviruses are the most common in children over this age.25 Other viruses detected are human bocavirus, adenovirus, human metapneumovirus and, less frequently, parainfluenza virus and influenza.20 In up to 30% of cases, 2 or more respiratory viruses are identified simultaneously, making it difficult to interpret their significance.20
BacteriaThe importance of bacteria in the etiology of CAP increases progressively with age, especially after the age of 5 years, when a bacterial pathogen can be identified in up to 30% of children in whom an etiological agent is detected.26
S. pneumoniae is the most common bacteria in children under 5 years of age, accounting for 30% of all cases,11 although its prevalence is decreasing progressively due to pneumococcal vaccination. The results of a recent study in Spain show that the introduction of the 13-valent pneumococcal vaccine (PCV13) has led to a 68% reduction in invasive pneumococcal disease, and serotypes 1 and 19A that previously accounted for 60% of all cases have all but disappeared.27 However, the rates of serotypes not included in this vaccine are beginning to increase rapidly, especially 8, 12 F and 9 N, in particular in adults.28
Other less common bacteria in CAP include: Streptococcus pyogenes (S. pyogenes), S. aureus, Moraxella catarrhalis and H. influenzae type b.
In children over 5 years of age hospitalized for CAP, Mycoplasma pneumoniae (M. pneumoniae) is the most frequently identified bacteria, requiring admission to intensive care in up to 10% of cases.29,30Chlamydia pneumoniae (C. pneumoniae) may also cause CAP in older children, although less frequently than M. pneumoniae.31
Most complicated CAPs, that involve pleural effusion, empyema, necrotizing pneumonia, or pulmonary abscess, are caused by non-vaccine serotypes of S. pneumoniae, followed by S. aureus and S. pyogenes.9
Community-acquired pneumonia in at-risk patientsPatients with underlying diseases have a higher incidence of pneumonia and a worse clinical course than healthy children. The etiological spectrum of CAP in this population varies according to the severity of anatomical and physiological alterations and the degree of immunosuppression. In general, the agents that usually cause CAP remain the most prevalent in these patients, but pulmonary infections with gram-negative bacilli, S. aureus and respiratory microorganisms of low virulence, such as non-typeable H. influenzae, are of greater importance.32
In children with more severe immunosuppression, opportunistic pathogens such as oral 〈-hemolytic streptococci, Pneumocystis jirovecii, Legionella pneumophila, cytomegalovirus and fungi should also be considered as possible etiological agents.33
In children with aspiration syndromes, pneumonia is usually caused by oral and oro/nasopharygeal aerobic and anaerobic flora, such as streptococci, Bacteroides and Fusobacterium species, and Prevotella melaninogenica.34
In patients with cystic fibrosis, S. aureus, Pseudomonas aeruginosa, non-typeable H. influenzae, and Burkholderia cepacia are common microorganisms.
A tuberculous etiology should be considered in children who have recently traveled to an endemic area or who have been in contact with a patient with active tuberculosis.35
In summary, age is the parameter that best predicts the etiology of CAP3 in childhood, although other factors, such as immune status, the presence of underlying disease, or the child's vaccination status, are also important (strong recommendation, high evidence).
Medical history and examinationSince the diagnosis is primarily clinical, an appropriate medical history and physical examination are essential to establish clinical suspicion and guide additional testing.36
Signs and symptoms related to the current disease should be recorded, and any possibly relevant factors (concomitant diseases, vaccination status, recent antibiotic use, day care, travel, exposure to infectious diseases, etc.) should be investigated.
The clinical presentation of CAP varies depending on age, causative agent, patient response, and extent of the disease, and both signs and symptoms are non-specific.37 Clinical manifestations are diverse and may occasionally be imperceptible, particularly in neonates and smaller infants.38
The presence of cough and fever, whether preceded or not by upper respiratory infection, is indicative of pneumonia, especially if associated with tachypnea and increased use of accessory muscles (chest indrawing), expiratory grunt, and nasal flaring.39 Other symptoms, such as chest or abdominal pain, diarrhea, vomiting, or headache due to meningism, sometimes occur, especially in the case of pneumonia located in the upper lobes.
Fever, the common sign of CAP in children, is a variable that has relatively low sensitivity and even less specificity.37 Although a pattern of lower, or absent, fever continues to be noted in pneumonia caused by viruses or atypical bacteria (C. pneumoniae, M. pneumoniae), compared to the high fever associated with a probable bacterial or mixed etiology (the latter being related with greater severity), the clinical value of this differentiation is not well established.37
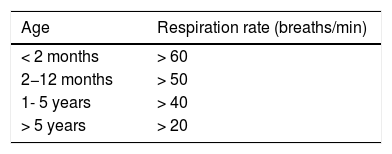
Like hypoxemia or intercostal chest indrawing, the presence of tachypnea (Table 2)40 may be a predictor of pneumonia in children, mainly in children under 3 years of age. A recent systematic review of 23 studies in children under 5 years of age concluded that respiratory rates (RR) higher than 40 breaths per minute are associated with an increased likelihood of radiological pneumonia, although the ability of RR to accurately distinguish children with and without pneumonia is very limited (likelihood ratio [LR] 1.5; 95% confidence interval [CI], 1.3–1.7).37 The severity of pneumonia (reflected by the level of hypoxemia) also correlates with the grade of tachypnea,41 especially in infants under 1 year of age with an RR greater than 70 bpm.42 In any case, it should be remembered that RR depends not only on age, but also on other factors, such as temperature, stress and anxiety, sleep, wakefulness, etc., and that, in the absence of tachypnea, the probability of pneumonia is very low.37
Definition of tachypnea.
| Age | Respiration rate (breaths/min) |
|---|---|
| < 2 months | > 60 |
| 2−12 months | > 50 |
| 1- 5 years | > 40 |
| > 5 years | > 20 |
Chest pain is a symptom usually reported by older children; in isolation it has little value in the diagnosis of pneumonia and is often associated with pleuritis or pleural effusion. The same applies to fever and cough as isolated parameters. Dry or productive cough, depending on the time since onset of the disease, may be absent at the beginning of pneumonia. When symptoms of upper respiratory tract infection associated with generalized wheezing and low fever predominate, bacterial pneumonia is unlikely.43
With regard to the physical examination, special attention should be paid to general condition, skin color (cyanosis, paleness), and respiratory distress.44
Bacterial pneumonia, as in the case of M. pneumoniae, may be accompanied by other symptoms,45 including cutaneous involvement and mucositis.46,47
Auscultation continues to be an essential component of the physical examination,48 although studies indicate that its findings do not correlate closely with radiological pneumonia, and may be normal in children. The subjectivity and technical difficulty involved in this procedure, especially in infants, probably contribute to its low diagnostic yield. The most common findings in pulmonary consolidations include rales, crackles, hypophonesis, and bronchial breath sounds. Wheezing, in addition to fine crackles in the bronchi, indicates bronchial involvement typical of bronchopneumonia, and points towards a viral origin or the presence of atypical bacteria such as M. pneumoniae or C. pneumoniae.44
Hypoxemia, often present without cyanosis, correlates positively with severity.37 Low levels of arterial oxygen saturation (SaO2) along with signs of increased work of breathing (chest retraction, nasal flaring, etc.) are the findings most robustly associated with the diagnosis of pneumonia.1 However, the diagnostic utility of these parameters is limited, as the LRs are less than 5 for both hypoxia and work of breathing: the LR for SaO2 ≤ 96% is 2.8 (95% CI, 2.1–3.6), with 64% sensitivity and 71% specificity,1 while the LR for SaO2 ≤ 95% is 3.5 (95% CI, 2.0–6.4) with lower sensitivity but higher specificity (96%).49 The LR for increased work of breathing is 2.1 (95% CI, 1.6–2.7).37
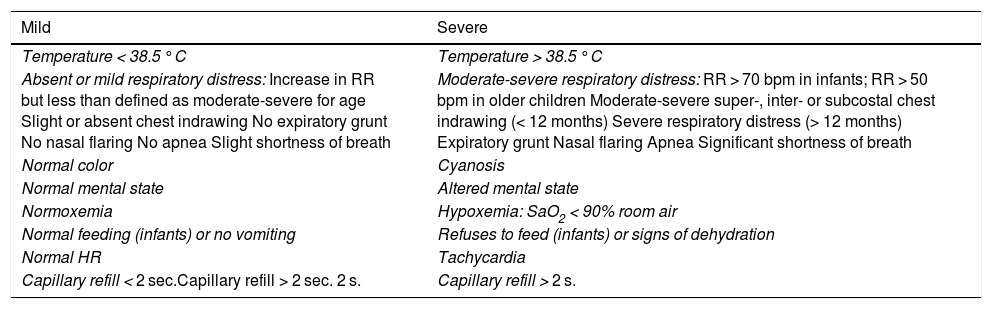
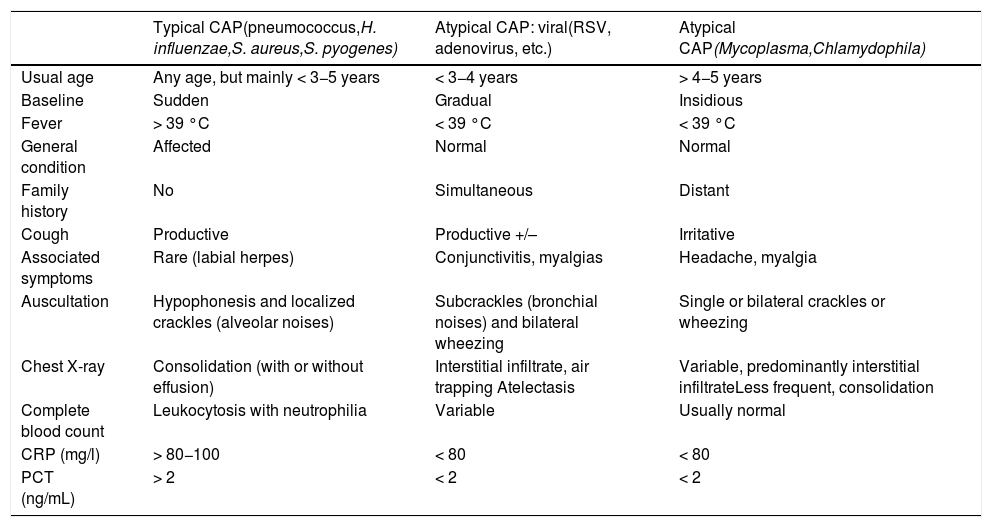
Classification of community acquired pneumoniasFrom a clinical point of view, CAP can be classified by severity (Table 3),2,50 and also as typical or atypical, albeit with exceptions, by combining clinical, radiological, and analytical characteristics (Table 4).51
Clinical classification of community-acquired pneumonias by severity.
| Mild | Severe |
|---|---|
| Temperature < 38.5 °C | Temperature > 38.5 °C |
| Absent or mild respiratory distress: Increase in RR but less than defined as moderate-severe for age Slight or absent chest indrawing No expiratory grunt No nasal flaring No apnea Slight shortness of breath | Moderate-severe respiratory distress: RR > 70 bpm in infants; RR > 50 bpm in older children Moderate-severe super-, inter- or subcostal chest indrawing (< 12 months) Severe respiratory distress (> 12 months) Expiratory grunt Nasal flaring Apnea Significant shortness of breath |
| Normal color | Cyanosis |
| Normal mental state | Altered mental state |
| Normoxemia | Hypoxemia: SaO2 < 90% room air |
| Normal feeding (infants) or no vomiting | Refuses to feed (infants) or signs of dehydration |
| Normal HR | Tachycardia |
| Capillary refill < 2 sec.Capillary refill > 2 sec. 2 s. | Capillary refill > 2 s. |
bpm: breaths/min; HR: heart rate; RR: respiration rate.
Characteristics of clinical, radiological and laboratory presentation associated with the etiology of community-acquired pneumonias.
| Typical CAP(pneumococcus,H. influenzae,S. aureus,S. pyogenes) | Atypical CAP: viral(RSV, adenovirus, etc.) | Atypical CAP(Mycoplasma,Chlamydophila) | |
|---|---|---|---|
| Usual age | Any age, but mainly < 3−5 years | < 3−4 years | > 4−5 years |
| Baseline | Sudden | Gradual | Insidious |
| Fever | > 39 °C | < 39 °C | < 39 °C |
| General condition | Affected | Normal | Normal |
| Family history | No | Simultaneous | Distant |
| Cough | Productive | Productive +/– | Irritative |
| Associated symptoms | Rare (labial herpes) | Conjunctivitis, myalgias | Headache, myalgia |
| Auscultation | Hypophonesis and localized crackles (alveolar noises) | Subcrackles (bronchial noises) and bilateral wheezing | Single or bilateral crackles or wheezing |
| Chest X-ray | Consolidation (with or without effusion) | Interstitial infiltrate, air trapping Atelectasis | Variable, predominantly interstitial infiltrateLess frequent, consolidation |
| Complete blood count | Leukocytosis with neutrophilia | Variable | Usually normal |
| CRP (mg/l) | > 80−100 | < 80 | < 80 |
| PCT (ng/mL) | > 2 | < 2 | < 2 |
CAP: Community-acquired pneumonia; CRP: C-reactive protein; PCT: procalcitonin; RSV: respiratory syncytial virus.
The diagnosis of CAP and its possible etiology depend on the integral assessment of the child's age, clinical and radiological signs, and the sum of certain clinical laboratory findings.
Blood testsBlood tests should not be performed routinely, as the white blood cell count and differential are not useful for determining the etiology of pneumonia.1 White blood cell counts greater than 15,000/mm3 with left shift are not clearly associated with a bacterial etiology, although C-reactive protein (CRP) values > 60−80 mg/mL do suggest this etiology.52
Procalcitonin (PCT) may be a potentially useful marker for making therapeutic decisions in emergency units2 or for guiding treatment.53 In fact, values < 0.25 ng/mL would rule out typical bacterial CAP (negative predictive value: 96%) and would help identify children who do not need antibiotic treatment.54 Some studies report that levels ≥ 1 ng/mL increase by up to 4-fold the probability of bacterial pneumonia55 and levels greater than 2 ng/mL have a specificity of 80% in predicting this etiology.56 However, there is no consensus on the usefulness of these biomarkers in determining microbial etiology in CAP,57 and there is no evidence that the use of a combination of these parameters (leukocytes, neutrophils, erythrocyte sedimentation rate [ESR], CRP and PCT) will increase sensitivity or specificity.58,59 PCT correlates positively with the severity of CAP2,60 and may be an indicator of the risk of bacteremia,60 but it is not useful in uncomplicated CAP.2
Other markers are being investigated61–64 and their clinical usefulness remains to be proven in coming years.
Microbiological diagnosisA definitive etiological diagnosis of CAP can only be established by isolating a pathogenic microorganism from a normally sterile fluid (blood, pleural fluid, lung biopsy). In children, this is only achieved in 30%-40% of cases and in less than 10% at disease onset, when this information would be useful for determining treatment. As a result of this low sensitivity, together with the poor cost-benefit ratio and the difficulty in obtaining adequate samples, microbiological testing not routinely recommended in previously healthy children with mild-moderate CAP who can be treated as outpatients.2
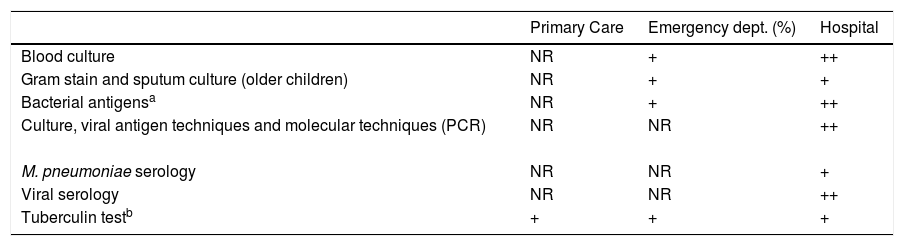
Rapid molecular diagnostic techniques (multiple PCR) are now available, significantly increasing diagnostic sensitivity in blood or pleural fluid samples,65 and, in the case of S. pneumoniae, distinguishing different serotypes involved in the disease. However, the results of multiplex panels should be interpreted with caution because they do not distinguish colonization from infection,66 nor can they be used alone to rule out a bacterial infection, given the possibility of concomitant viral-bacterial infection, particularly in more severe cases.67 Microbiological testing should be reserved for the following situations: pneumonia associated with bacteremia or pleural effusion; immunosuppression or immunosuppressive treatment; moderate-severe or slow-progressing pneumonia; and epidemic outbreaks.1 In these cases, the diagnostic tests to be used would be2: blood culture; Gram stain and sputum culture, PCR detection of virus or immunofluorescence in nasopharyngeal secretions or nasal swabs; serology, for respiratory viruses, M. pneumoniae, and C. pneumoniae, and, if a pleural fluid sample is available, direct microscopy, culture (low sensitivity), detection of pneumococcal antigen (sensitivity and specificity > 90%), and PCR for S. pneumoniae.
A predominant microorganism or intracellular organisms detected in an appropriate sputum sample (≤ 10 epithelial cells and ≥ 25 polymorphonuclear leukocytes [⋅100]) is indicative of the etiological agent.68 In these cases, Gram stain has a sensitivity and specificity for pneumococcus of 85% and 62%, respectively. However, the diagnostic yield of induced sputum is not sufficient to include this procedure in the routine diagnosis of CAP.69Nasopharyngeal aspirates or exudate are used for the detection of Bordetella pertussis and respiratory viral antigens (enzyme immunoassay and immunochromatography), but not for bacterial cultures, as the causative bacteria may be the same as those of the normal oropharyngeal flora.70 In hospitalized children, the study of respiratory viruses in nasopharyngeal secretions may be helpful (strong recommendation, high evidence).
The same is true of the detection of pneumococcal urinary antigens, which can be positive in children < 4−5 years old, pneumococcal carriers, or patients who have recently received a pneumococcal vaccine.1,2,71,72 After this age, pneumococcal urinary antigen has a similar value to adults72 and may be useful as a negative predictor of pneumococcal infection in older children.2 Detection of Legionella antigen in urine should be considered in cases with clinical and epidemiological suspicion of legionellosis.
Serological diagnosis should not be included as a routine test since it requires the comparison of 2 samples at an interval of 2–4 weeks (acute phase and convalescence), so is not helpful in decision-making, and the seroconversion of some pathogens is also difficult to assess. Even so, the British guidelines propose storing serum samples in the acute phase and taking a sample during convalescence in cases where a microbiological diagnosis was not reached during the acute stage, and recommend this procedure for the diagnosis of atypical and respiratory virus infections.2
Other tests, such as the tuberculin test or Interferon-Gamma Release Assays (IGRA), are not recommended in all pneumonias. They are justified only in the case of clinical or epidemiological suspicion of tuberculosis, refractory pneumonia, or in marginal settings, and travel and migration of the population from areas of high prevalence in tuberculosis. Table 4 shows etiological, clinical and laboratory correlations, and Table 5 shows the microbiological tests proposed at each level of care.
Microbiological tests proposed for each level of care in the study of community-acquired pneumonia.
| Primary Care | Emergency dept. (%) | Hospital | |
|---|---|---|---|
| Blood culture | NR | + | ++ |
| Gram stain and sputum culture (older children) | NR | + | + |
| Bacterial antigensa | NR | + | ++ |
| Culture, viral antigen techniques and molecular techniques (PCR) | NR | NR | ++ |
| M. pneumoniae serology | NR | NR | + |
| Viral serology | NR | NR | ++ |
| Tuberculin testb | + | + | + |
NR: not recommended; PCR: polymerase chain reaction; +: recommended; ++: strongly recommended.
Studies to determine the etiology of community-acquired pneumonia in children only establish the cause in 20%-50% of cases2 (moderate recommendation, moderate evidence).
Chest X-rayChest X-ray is the gold standard for establishing a diagnosis of pneumonia, but since it does not modify therapeutic decisions or improve clinical outcomes,73 it is not mandatory in clinical guidelines for CAP,1,2 and may be dispensed with in previously healthy children with a first episode of pneumonia and no severity criteria, or in children with fever but no tachypnea, unless warranted by the patient data3 (strong recommendation, high evidence). Indications are given in Table 6.12
Indications for chest X-ray if community-acquired pneumonia is suspected in a child.
| Severe disease or suspected complications |
|---|
| Unclear clinical diagnosis (inconclusive signs/symptoms, children with prolonged/ unexplained fever and leukocytosis) |
| Hospital admission (to assess presence/characteristics of infiltrates and potential complications) |
| Previous episodes of pneumonia |
| Poor response to antibiotic treatment, slow progress |
| Rule out alternative diseases (foreign body aspiration, heart failure, etc.) |
In most cases, frontal projection is usually sufficient for the diagnosis of CAP.2 Lateral projection increases the radiation dose and does not usually provide significant information. As such, it should be reserved for inconclusive cases, diagnostic doubts, or suspected lymphadenopathies1,2 and not routinely performed (strong recommendation, high evidence). The radiological expression of pneumonia is consolidation or pulmonary parenchymal infiltrate, with 2 characteristic radiologic patterns (alveolar and interstitial) that, while conventionally associated individually with a type of infection (bacterial/viral or M. pneumoniae), are not unique to any specific etiology.55 The alveolar pattern is characterized by lobar or segmental consolidation, with or without air bronchogram or alveologram. The interstitial pattern is caused by bilateral, diffuse and irregular parahiliar infiltrates, air trapping or segmental atelectasis due to mucosal plugs and peribronchial thickening, and can be observed in viral pneumonias or those caused by M. pneumoniae, C. pneumoniae and Legionella sp. There is also a mixed pattern, which combines the above characteristics and is another, not uncommon, form of presentation of CAP.74
An image of “round pneumonia” is indicative of pneumococcal infection73 and the presence of pneumatoceles, cavities, or large pleural effusion is associated with bacterial pneumonia. The first radiological sign of effusion is usually the occupation of the costophrenic angle, which, when it appears, already indicates the existence of a major effusion; therefore, if this complication is suspected an ultrasound should be performed to evaluate the amount of fluid present and decide on the action to take.2
In summary, analytical and radiological studies cannot be used to establish the viral or bacterial etiology of pneumonia in children with absolute certainty. In routine clinical practice, the results of microbiological and laboratory tests should always be interpreted together with the medical history, physical examination findings, and chest X-ray75 (moderate recommendation, moderate evidence).
Chest ultrasoundCurrent evidence supports ultrasound as a useful imaging alternative for the diagnosis and follow-up of pneumonia in children76–79 (moderate recommendation, moderate evidence), because it is readily available, quick to learn, easy to perform, affordable, and radiation-free.80 Some authors believe that it should take precedence over radiography.81 Two recent meta-analyses80,82 confirm its high sensitivity (93%-94%), specificity (93%-96%), and area below the curve (0.98). Ultrasound provides data on the lung parenchyma: bronchogram (distorted or normal), homogeneity or heterogeneity of consolidation, areas with avascularity or low echogenicity due to necrosis, vascularized wall areas associated with the formation of abscesses, etc.77,83 It is much more sensitive than radiography for confirming minimal effusions84–86 and provides more information than computed tomography (CT) in terms of the amount, nature of the effusion (septated or not), and location of the puncture site, if necessary.
Computed tomographyCT may be indicated if complications are suspected (severe or complex pneumonia, pneumonia in immunocompromised patients, antibiotic-refractory pneumonia, recurrent or unresolved pneumonia, patients with clinical suspicion of pneumonia but normal or questionable chest X-ray findings, and pneumonia with suspected underlying diseases87), or if there is difficulty in differentiating CAP from other diseases.86 It is very useful for assessing the pulmonary parenchyma, and defines necrotic lesions, cavities, pneumatoceles, abscesses or bronchopleural fistulas with great precision. It also complements the ultrasound evaluation of empyema, locating the drainage tube, and evaluating possible parenchymal reexpansion failures after tube placement.86,88
Magnetic resonance imagingAlthough recent studies suggest that magnetic resonance imaging (MRI) is slightly more sensitive than X-ray in detecting pneumonia, its high cost, limited availability, and the meagre radiation dose that would be avoided if used instead of X-ray rule it out as a first-line diagnostic option.88,89 It may be considered in children with complicated pneumonia, replacing repeated CT scans as a follow-up test, or in patients with chronic diseases who will undergo multiple CT scans throughout their life, as a method for evaluating the lungs without increasing the radiation dose.88
Bronchoscopy and related techniquesFiberoptic bronchoscopy (FB) is reserved for cases of severe or potentially severe CAP, slow progress, or persistent radiological abnormalities, and for children with recurrent pneumonia in the same site.90 It is also indicated in oncological or immunocompromised patients who do not respond adequately to initial treatment, and in whom the causative agent must be identified.91 In these patients, the yield is higher (80%) than in immunocompetent children, in whom the isolation rate may be higher if the procedure is performed early.92
FB should always be accompanied by bronchoalveolar lavage (BAL) at one or more sites for cell culture and analysis.92 The isolation of certain microorganisms, such as Mycobacterium tuberculosis (M. tuberculosis), RSV, influenza virus or M. pneumoniae, is sufficient for them to be considered the etiological cause of the pneumonia, although they may not be the only causative agents involved. The detection of other bacteria (S. pneumoniae, H. influenzae, etc.) that typically occur in the oropharyngeal flora could indicate contamination, and in these cases quantitative cultures should be used, with a colony-forming unit per milliliter (CFU/mL) ≥ 104 indicating infection. Bronchial brushing has a lower diagnostic yield than BAL, so its use in the pediatric setting has declined.93
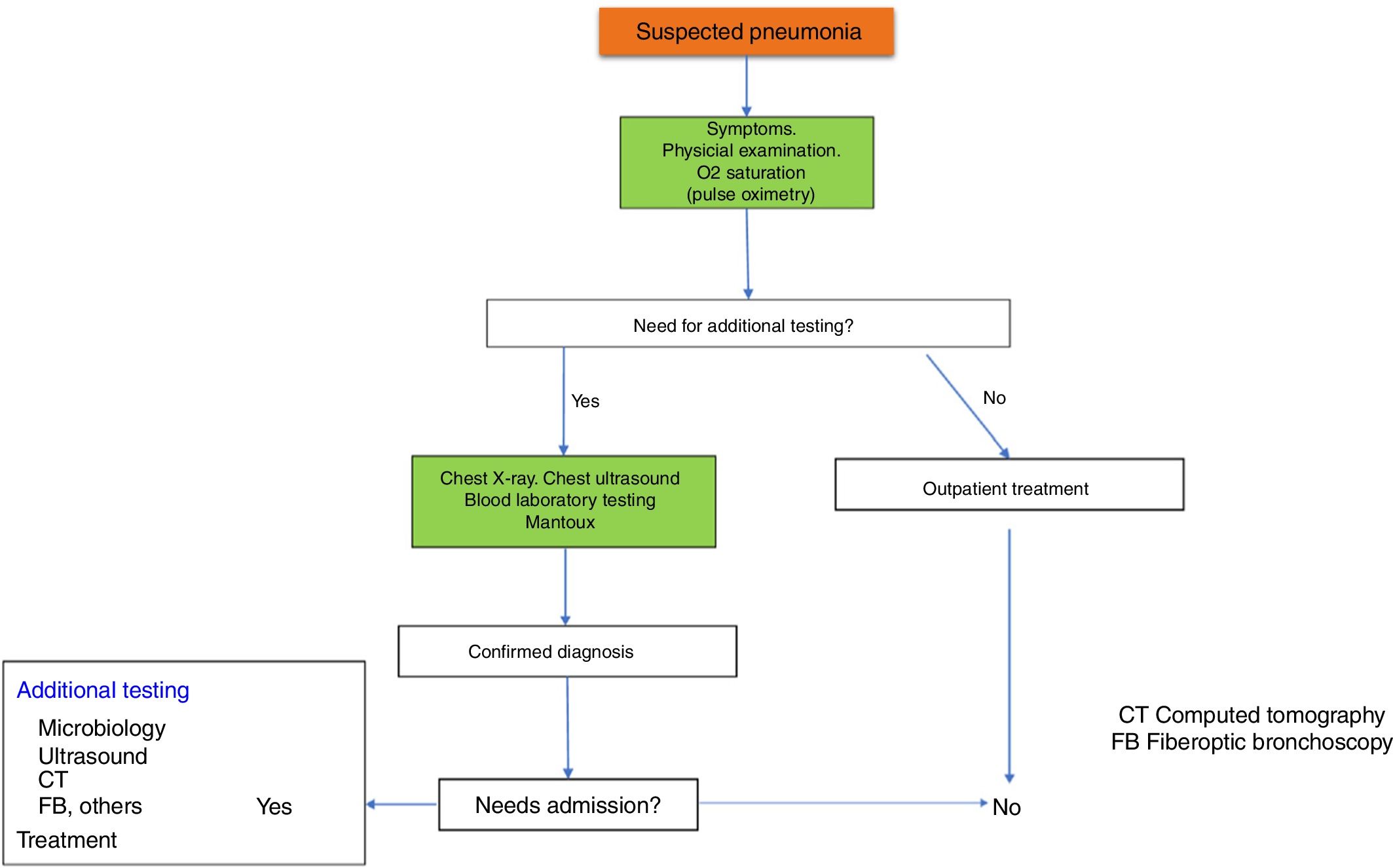
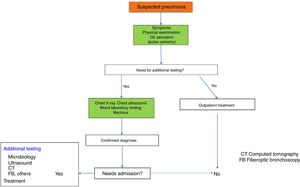
Fig. 1 shows the diagnostic algorithm for suspected CAP.
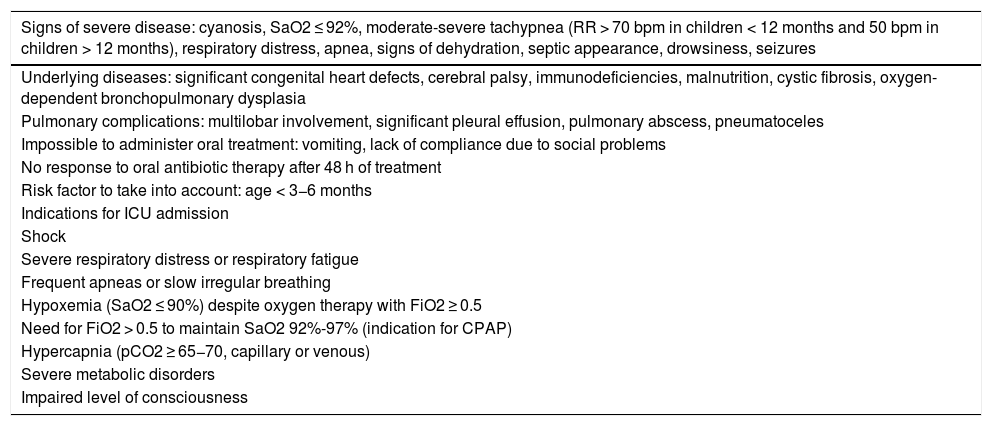
Disease course and monitoring of community-acquired pneumoniasAfter pneumonia has been diagnosed, its severity must be evaluated and decisions taken on whether hospitalization is needed. In uncomplicated cases, 90% of patients will become afebrile within 48 h of starting antibiotic treatment, and only a small proportion will require hospital admission (Table 7).50,94 In the community setting, the pediatrician should perform a clinical assessment 48 h after pneumonia has been diagnosed and treatment started.1,2
Indications for hospital and ICU admission of children with community-acquired pneumonia.50,94.
| Signs of severe disease: cyanosis, SaO2 ≤ 92%, moderate-severe tachypnea (RR > 70 bpm in children < 12 months and 50 bpm in children > 12 months), respiratory distress, apnea, signs of dehydration, septic appearance, drowsiness, seizures |
|---|
| Underlying diseases: significant congenital heart defects, cerebral palsy, immunodeficiencies, malnutrition, cystic fibrosis, oxygen-dependent bronchopulmonary dysplasia |
| Pulmonary complications: multilobar involvement, significant pleural effusion, pulmonary abscess, pneumatoceles |
| Impossible to administer oral treatment: vomiting, lack of compliance due to social problems |
| No response to oral antibiotic therapy after 48 h of treatment |
| Risk factor to take into account: age < 3−6 months |
| Indications for ICU admission |
| Shock |
| Severe respiratory distress or respiratory fatigue |
| Frequent apneas or slow irregular breathing |
| Hypoxemia (SaO2 ≤ 90%) despite oxygen therapy with FiO2 ≥ 0.5 |
| Need for FiO2 > 0.5 to maintain SaO2 92%-97% (indication for CPAP) |
| Hypercapnia (pCO2 ≥ 65−70, capillary or venous) |
| Severe metabolic disorders |
| Impaired level of consciousness |
bpm: breaths/min; CPAP: continuous positive airway pressure; FiO2: inspired fraction of oxygen; ICU: Intensive Care Unit; pCO2: partial pressure of carbon dioxide; RR: respiration rate; SaO2: arterial oxygen saturation.
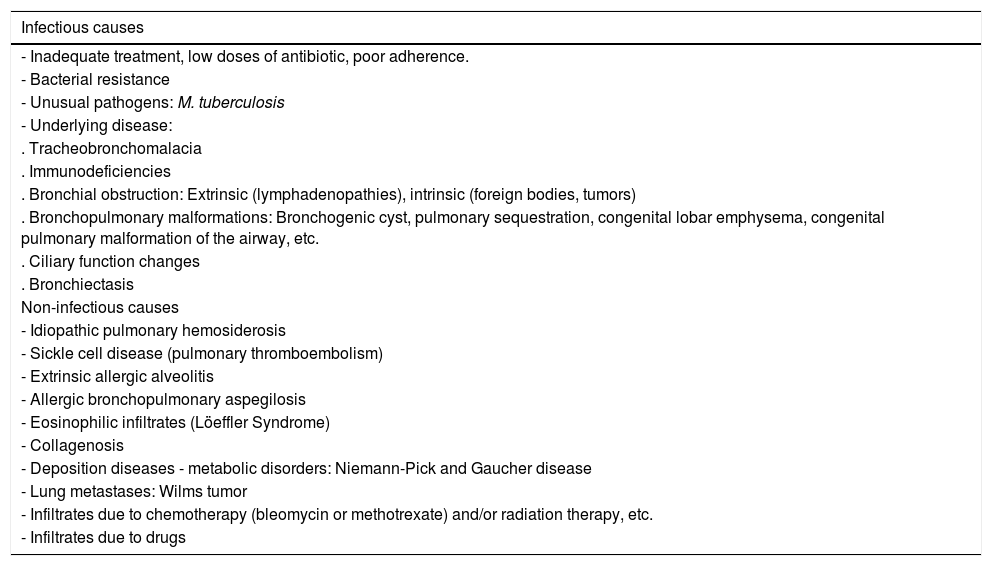
If, contrary to expectations, there is no significant improvement after 48−72 hours of treatment (persistence of fever, worsening general condition, dyspnea, etc.), the reasons for this should be considered, such as incorrect diagnosis, ineffective treatment (poor compliance or incorrect dose, presence of resistance), appearance of complications (necrotizing pneumonia, parapneumonic effusion, pulmonary abscess), viral etiology or a rarer causative microorganism (M. tuberculosis, Actinomyces sp., fungi, protozoa), previously undiagnosed immunodeficiency, non-infectious cause and associated bronchial obstruction, underlying process such as congenital lung malformation, or non-infectious disease: pulmonary hemorrhage, pulmonary edema, diaphragmatic hernia, eosinophilic pneumonia, organizational pneumonia, pulmonary thromboembolism. All these possible causes must be evaluated and explored (Table 8).50,94
Most common causes of persistent pneumonia.104.
| Infectious causes |
|---|
| - Inadequate treatment, low doses of antibiotic, poor adherence. |
| - Bacterial resistance |
| - Unusual pathogens: M. tuberculosis |
| - Underlying disease: |
| . Tracheobronchomalacia |
| . Immunodeficiencies |
| . Bronchial obstruction: Extrinsic (lymphadenopathies), intrinsic (foreign bodies, tumors) |
| . Bronchopulmonary malformations: Bronchogenic cyst, pulmonary sequestration, congenital lobar emphysema, congenital pulmonary malformation of the airway, etc. |
| . Ciliary function changes |
| . Bronchiectasis |
| Non-infectious causes |
| - Idiopathic pulmonary hemosiderosis |
| - Sickle cell disease (pulmonary thromboembolism) |
| - Extrinsic allergic alveolitis |
| - Allergic bronchopulmonary aspegilosis |
| - Eosinophilic infiltrates (Löeffler Syndrome) |
| - Collagenosis |
| - Deposition diseases - metabolic disorders: Niemann-Pick and Gaucher disease |
| - Lung metastases: Wilms tumor |
| - Infiltrates due to chemotherapy (bleomycin or methotrexate) and/or radiation therapy, etc. |
| - Infiltrates due to drugs |
In clearly deteriorating cases, hospitalization is necessary to administer intravenous (IV) antibiotic treatment and any necessary support, and to perform complementary examinations to evaluate the existence of potential complications and clarify the etiology (new chest X-ray, ultrasound, blood tests, ESR, PCR, blood culture, viral antigen detection, serology, tuberculin testing, FB, BAL, CT, etc.).50
If there is no clear deterioration, but no significant improvement is observed within 72−96 hours, antibiotic resistance, presence of another pathogen, or a non-infectious cause of pneumonia must be considered. If resistance to antibiotics or other pathogens is suspected, the administration of another antibiotic to expand coverage against S. pneumoniae and atypical bacteria must be considered. There is no specific waiting time, but if after 72 h there is no clear improvement, the above options should be considered.94
However, with proper treatment, clinical progress is favorable in most cases.95 Residual cough may persist for a few weeks, especially after viral or M. pneumoniae-associated CAP.96 Recovery is usually complete and without sequelae in previously healthy children so, in general, no clinical laboratory monitoring is necessary when the course is normal and the patient remains asymptomatic.97
It is important to remember that although clinical resolution is usually rapid in most cases, radiological changes usually take 3−7 weeks to normalize. The various guidelines and consensus documents specify that routine radiological follow-up is not necessary in patients who remain asymptomatic after uncomplicated CAP.97 In contrast, a follow-up X-ray is justified in the following cases: persistent symptoms, history of recurrent pneumonia, presence of atelectasis, round pneumonia, empyema, pneumatocele, pulmonary abscess, or other concomitant disease.1,2,50,94,98
If the pneumonia required hospital admission (Table 7), follow-up after discharge may be conducted by the primary care pediatrician or in the hospital, depending on the reason for admission, severity, complications, etc.
With regard to clinical course, 2 other possibilities in addition to rapid cure must be taken into account, namely slowly resolving pneumonia, in which the cure process is slower than usual, but clinical and radiological normalization is finally achieved, and unresolved or persistent pneumonia, defined as clinical or radiological symptoms persisting over a certain period (≥ 1 month), despite the administration of antibiotic treatment for 10 days.99,100 Few authors specify the time after which pneumonia should be considered persistent, but this ranges from 1 to 3 months. The real incidence is unknown since cases of persistent and recurrent pneumonia overlap in the published series.101,102
In the case of slowly resolving or persistent CAP, once normal prolonged radiological resolution has been ruled out, the possible causes of a hypothetical anti-infectious treatment failure must be raised, without forgetting the possibility of a non-infective etiology of a persistent radiological image103–106 (Table 8). In all these cases, FB, with or without BAL, combined with other studies (immunological, imaging, etc.), has been shown to help elucidate the underlying cause.106,107
TreatmentCurrent status of antimicrobial resistanceBacteria that are potential causes of CAP and which may have antimicrobial resistance problems include: S. pneumoniae, S. aureus and S. pyogenes. In our setting, other agents, such as M. pneumoniae, C. pneumoniae, or viruses, do not usually present problems of this type. M. pneumoniae and C. pneumoniae are usually susceptible to macrolides and the only virus that is a candidate for antiviral treatment, the influenza virus, has not so far shown significant resistance to oseltamivir in Spain108,109 (personal communication: Microbiology Department of the Hospital Vall d’Hebron, Report entitled “Vigilància virològica dels virus de la grip a Barcelona ciutat: informe final de temporada 2018–2019”, authors: Andrés Antón and Diego van Esso, Hospital Vall d’Hebron and Barcelona Territorial Management of the Catalan Institute of Health).
The most reliable data on the resistance of the major respiratory pathogens in our setting are provided periodically by the national multicenter Community-based Antibiotic Susceptibility Project in Spain (SAUCE) study. This study, published in 2010 as the SAUCE-4 project,110 provides susceptibility and resistance results according to official CLSI breakpoints. It comprises a total of 2559 S. pneumoniae isolates, 2287 S. pyogenes and 2287 H. influenzae, and figures are compared with those recorded in the previous 11 years. In short, practically all circulating strains of S. pneumoniae in Spain, in terms of beta-lactam sensitivity, are currently sensitive to oral (PO) amoxicillin, penicillin, and ampicillin IV. All are also sensitive to cefotaxime.
These data, based on samples obtained 6–7 years ago from children and adults, closely mirror the recent findings of the study on pneumococcal susceptibility to antibiotics conducted in the Community of Madrid (Heracles study) (May 2011-April 2013)111 in which all S. pneumoniae strains (100%) isolated from children under 15 years of age with invasive non-central nervous system pneumococcal disease—including bacterial pneumonia and empyema—were susceptible to penicillin and cefotaxime.
In recent years, there has been no significant change in the susceptibility patterns reported in the SAUCE-4 study, with the single exception of a significant reduction in S. pyogenes resistance to macrolides in some regions, which, in the province of Barcelona, is consistently lower than 10%.108
On the other hand, the increase in methicillin-resistant S. aureus (MRSA) infections in the community reported in some studies in our setting, with percentages ranging from 5% in Malaga to 12.5% in Badalona between 2008 and 2011,112 and 16% in Barcelona in 2017113 (personal communication: Montse Giménez, Microbiology Department of the Hospital Universitari Germans Trias i Pujol, and N. Larrosa, Microbiology Department of the Hospital Universitari Vall d’Hebron) means that we must take this microorganism and its resistance pattern into consideration when treating severe pneumonias; to date, all strains remain sensitive to vancomycin. In a recent study involving pediatric and adult populations, conducted in emergency departments in 7 European countries, with Spanish participation, the mean community-associated MRSA rate was 15%, with a clear north-to-south increase in resistance114 (personal communication: N. Larrosa, Microbiology Department, Universitari Vall d’Hebron Hospital).
Antibiotic treatment of community-acquired pneumonia and complicationsEmpirical treatment of CAP and its complications is established according to the common pathogens. However, one of the most important problems is the correct distinction between cases of probable viral etiology and cases of probable bacterial etiology. In general, antibiotic treatment is indicated in cases of typical CAP where bacterial etiology is suspected. In cases of atypical CAP, antibiotic therapy will only be indicated in patients older than 4−5 years, whereas in children under this age antibiotic treatment will not be prescribed unless they meet some of the criteria described below.115
If the decision is taken to start outpatient antibiotic treatment in typical CAP, taking into account that most are caused by S. pneumoniae, almost all of which are currently susceptible to penicillin and amoxicillin, the antibiotic of choice is amoxicillin PO, at a dose of 80 mg/kg/day, every 8 h (Table 9)114,116–119 (strong recommendation, high evidence). The use of amoxicillin-clavulanic acid PO (80 mg/kg/day) would only be justified in patients with underlying diseases predisposing them to a wider range of bacteria, pulmonary aspiration, or incomplete H. influenzae type b vaccination (strong recommendation, high evidence). The recommended duration of treatment in a patient with a typical uncomplicated CAP that does not require admission is a maximum of 7 days.119
Antibiotic treatment of children with community-acquired pneumonia.
| Outpatient treatment | |
|---|---|
| Typical pneumonia | Amoxicillin orally 80 mg/kg/day divided into 3 doses (every 8 h). Maximum 6000 mg/day, 7 days |
| Atypical pneumonia caused by Mycoplasma or Chlamydophilia | Azithromycin orally 10 mg/kg/ every 24 h (maximum 500 mg/day, 3 days) |
| Clarithromycin orally 15 mg/kg/day, every 12 hours, maximum 1000 mg/day, 7 days | |
| Inpatient treatment | |
| Typical pneumonia with no parapneumonic pleural effusion | Equally valid options:Ampicillin IV: 150−200 mg/kg/day, every 6 h (maximum 12 g/day)Penicillin G sodium IV: 250,000−300,000 IU/kg/day, every 4 h (maximum 24 million IU/day) |
| Typical pneumonia with parapneumonic pleural effusion | Equally valid options:Ampicillin IV: 250−300 mg/kg/day, every 6 h (maximum 12 g/day)Penicillin G sodium IV: 300,000−400,000 IU/kg/day, every 4 h (maximum 24 million IU/day) |
| Typical CAP in severe patient (PICU) | Cefotaxime (200−300 mg/kg/day, every 6 h; max. 12 g/day)+one of the followinga:cloxacillin IV (150−200 mg/kg/day, every 6 h; max. 6 g/day)orclindamycin IV (30−40 mg/kg/day, every 6−8 h; max. 4.8 g/dayb)orcloxacillin IV (60 mg/kg/day, every 6 h; max. 4 g/dayc)±macrolide IV (options: azithromycin 10 mg/kg/day, every 24 h; clarithromycin 15 mg/kg/day, every 12 h) |
| CAP with interstitial pattern in severe patient (PICU) | Cefotaxime (200 mg/kg/day) + macrolide IV (clarithromycin or azithromycin)±Cotrimoxazole IV (20 mg trimethoprim/kg/day, every 6 hd) |
CAP: community-acquired pneumonia; IV: intravenous; MRSA: methicillin-resistant Staphylococcus aureus; PICU: Pediatric Intensive Care Unit.
In the case of atypical CAP in children under 4−5 years of age, etiology is usually viral, so in principle antibiotics will not be prescribed. In those older than 4−5 years, in whom an etiology of M. pneumoniae is rare and C. pneumoniae even rarer, the use of macrolides PO is recommended120 (Table 9) (strong recommendation, high evidence).
If a properly vaccinated patient needs to be admitted to hospital, and pneumococcal etiology is assumed or confirmed, the antibiotic of choice in typical CAP is currently high-dose penicillin G sodium or ampicillin IV, given the excellent tolerance (Table 9)112 (strong recommendation, high evidence).
In children under 6 months of age with typical CAP who will not yet have completed their initial Hib vaccination, admission is recommended depending on their general condition, degree of hypoxemia, etc., for IV treatment with amoxicillin-clavulanic acid or cefuroxime.114 In children under 3 months, empirical treatment consists of ampicillin and cefotaxime.114
In cases associated with influenza, the most frequent superinfection is S. pneumoniae and, to a lesser extent, S. aureus, S. pyogenes and H. influenzae, so empirical therapy with amoxicillin-clavulanic acid may be appropriate. MRSA co-infection has been found to be more severe, so when a patient with concomitant influenza and severe pneumonia is admitted to the pediatric ICU, empirical use of clindamycin (or vancomycin, depending on local susceptibility data) together with a cephalosporin (cefuroxime or cefotaxime) may be appropriate. In immunocompromised patients or patients with other risk factors who develop severe respiratory infection due to the influenza virus, treatment with an antiviral (usually oseltamivir) should be initiated early, given the greater severity of influenza virus infections in hosts this of type.120
If aspiration is suspected, amoxicillin-clavulanic acid is recommended.115
Complicated forms of CAP, such as necrotizing pneumonia and pulmonary abscess, are usually initially treated empirically with ampicillin or cefotaxime combined with clindamycin for 4 weeks, or for at least 2 weeks after fever has disappeared118 and, if the etiological agent is known, the antibiotic therapy may be subsequently adjusted.
In case of pleural empyema, a dose increase is recommended to reach higher concentrations in the pleural space (Table 9).114 When the patient has remained afebrile for 24−48 hours and once the pleural tube has been removed, treatment can be switched to the oral route.116 The total duration of treatment is usually 2–4 weeks.118
Antibiotic treatment in special situationsSevere typical CAP, with or without pleural effusionThese patients are usually admitted to intensive care. The etiological spectrum is broader (S. pneumoniae, S. aureus, S. pyogenes) (Table 9). Cefotaxime is recommended and may be associated with an antibiotic with antistaphylococcal activity such as cloxacillin112 (strong recommendation, high evidence). If the prevalence of MRSA in the environment is > 10%, depending on local susceptibility data, antibiotics such as clindamycin or vancomycin should be used.115 Second-line antibiotics for which little experience is available in children, e.g., linezolid, ceftaroline, telavancin, or quinupristin-dalfopristin, should be used only in MRSA infections that do not respond to these antistaphylococcal compounds.118 Combination with a macrolide has been shown to improve survival in severe cases in adults, so its administration could be assessed, although there is still a lack of consistency in the data in children.121–124
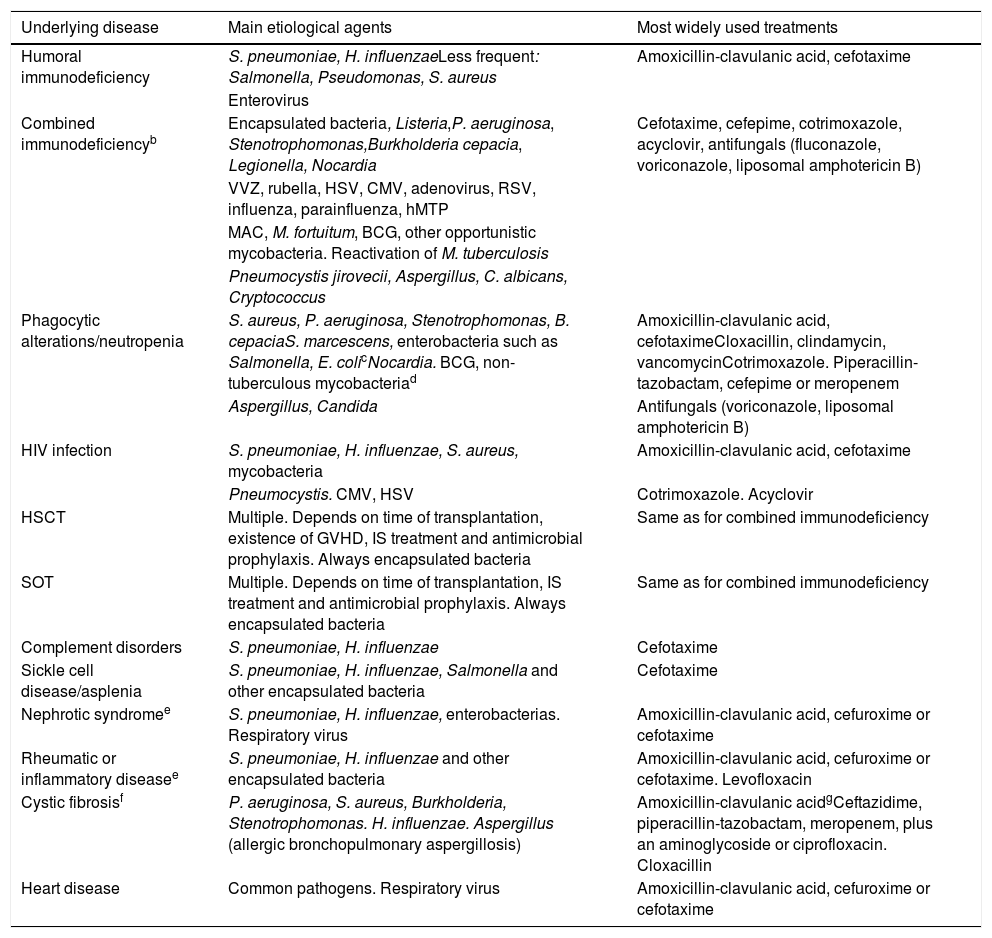
Underlying diseases or immunosuppressionIn children with mild-moderate immunosuppression, amoxicillin-clavulanic acid, cefuroxime, or cefotaxime is recommended as empirical treatment,115 with the possibility of adding a neuraminidase inhibitor (oseltamivir) if the influenza virus is detected in respiratory secretions. If S. aureus is suspected, cefotaxime would not be optimal, and if MRSA is suspected in the community, it should be combined with clindamycin or vancomycin, depending on the sensitivity of local isolates. Adding a macrolide should be evaluated in case of diffuse pulmonary infiltrates, and even cotrimoxazole should be considered if Pneumocystis is suspected.115Table 10 shows the most important immunodeficiencies and their general antimicrobial management.
Microorganisms commonly associated with pneumonia in children with some serious underlying diseasesa and most widely used treatments (prepared by the authors).
| Underlying disease | Main etiological agents | Most widely used treatments |
|---|---|---|
| Humoral immunodeficiency | S. pneumoniae, H. influenzaeLess frequent: Salmonella, Pseudomonas, S. aureus | Amoxicillin-clavulanic acid, cefotaxime |
| Enterovirus | ||
| Combined immunodeficiencyb | Encapsulated bacteria, Listeria,P. aeruginosa, Stenotrophomonas,Burkholderia cepacia, Legionella, Nocardia | Cefotaxime, cefepime, cotrimoxazole, acyclovir, antifungals (fluconazole, voriconazole, liposomal amphotericin B) |
| VVZ, rubella, HSV, CMV, adenovirus, RSV, influenza, parainfluenza, hMTP | ||
| MAC, M. fortuitum, BCG, other opportunistic mycobacteria. Reactivation of M. tuberculosis | ||
| Pneumocystis jirovecii, Aspergillus, C. albicans, Cryptococcus | ||
| Phagocytic alterations/neutropenia | S. aureus, P. aeruginosa, Stenotrophomonas, B. cepaciaS. marcescens, enterobacteria such as Salmonella, E. colicNocardia. BCG, non-tuberculous mycobacteriad | Amoxicillin-clavulanic acid, cefotaximeCloxacillin, clindamycin, vancomycinCotrimoxazole. Piperacillin-tazobactam, cefepime or meropenem |
| Aspergillus, Candida | Antifungals (voriconazole, liposomal amphotericin B) | |
| HIV infection | S. pneumoniae, H. influenzae, S. aureus, mycobacteria | Amoxicillin-clavulanic acid, cefotaxime |
| Pneumocystis. CMV, HSV | Cotrimoxazole. Acyclovir | |
| HSCT | Multiple. Depends on time of transplantation, existence of GVHD, IS treatment and antimicrobial prophylaxis. Always encapsulated bacteria | Same as for combined immunodeficiency |
| SOT | Multiple. Depends on time of transplantation, IS treatment and antimicrobial prophylaxis. Always encapsulated bacteria | Same as for combined immunodeficiency |
| Complement disorders | S. pneumoniae, H. influenzae | Cefotaxime |
| Sickle cell disease/asplenia | S. pneumoniae, H. influenzae, Salmonella and other encapsulated bacteria | Cefotaxime |
| Nephrotic syndromee | S. pneumoniae, H. influenzae, enterobacterias. Respiratory virus | Amoxicillin-clavulanic acid, cefuroxime or cefotaxime |
| Rheumatic or inflammatory diseasee | S. pneumoniae, H. influenzae and other encapsulated bacteria | Amoxicillin-clavulanic acid, cefuroxime or cefotaxime. Levofloxacin |
| Cystic fibrosisf | P. aeruginosa, S. aureus, Burkholderia, Stenotrophomonas. H. influenzae. Aspergillus (allergic bronchopulmonary aspergillosis) | Amoxicillin-clavulanic acidgCeftazidime, piperacillin-tazobactam, meropenem, plus an aminoglycoside or ciprofloxacin. Cloxacillin |
| Heart disease | Common pathogens. Respiratory virus | Amoxicillin-clavulanic acid, cefuroxime or cefotaxime |
BCG: bacillus Calmette-Guérin; CAP: community-acquired pneumonia; CMV: cytomegalovirus; hMTP: Human metapneumovirus; HSCT: hematopoietic stem cell transplantation; HSV: herpes simplex virus; IF-γ: gamma inferon; IL-12: interleukin 12; IS: Immunosuppressive; MAC: Mycobacterium avium-complex; RSV: respiratory syncytial virus; SOT: solid organ transplant; TB: tuberculosis; VVZ: varicella zoster virus.
Microorganisms that produce CAP in healthy children should always be considered, especially respiratory viruses and S. pneumoniae, and, to a lesser extent, H. influenzae. The degree of immunosuppression, the immediate history (recent hospitalization, chemoprophylaxis, previous vaccines) and the clinical situation of the patient should together guide the clinician with respect to the aggressiveness of the therapeutic approach (need for admission, invasive diagnostic and microbiological tests, combination of antibiotics, use of antivirals and antifungals).
Depressed cell-mediated immunity could lead to Strongyloides stercoralis hyperinfection (rule out in case of severe eosinophilia; it is important to know the patient's country of origin).
Assess the importance of endemic infections in the country of origin; the incidence of TB in immigrant adults with blood cancers has been seen to be very high.
Especially important in IF-© and IL-12 receptor deficiency, where Salmonella and Listeria may also be involved.
Will depend largely on the immunosuppressive treatment received and occur in infections due to opportunistic pathogens. For example, administration of anti-TNF and corticosteroids at immunosuppressive doses has been associated with reactivation of tuberculosis and infections with Cryptococcus, Aspergillus, Listeria, Pneumocystis, HSV, VVZ and CMV, among others.
Recommendations are given in Table 11.117
Clinical classification of community-acquired pneumonias by severity.
| Late, non-severe allergic reaction | Immediate or late severe allergic reaction | |
|---|---|---|
| Outpatient treatment | Cefuroxime-axetil PO 30 mg/kg/day (adult dose: 500 mg every 8 h) | Alternatives:Azithromycin PO 10 mg/kg/day 3 days (adult dose: 500 mg/24 h first day, followed by 250 mg/24 h 4 days: or 500 mg/24 h 3 days)Clindamycin PO 30 mg/kg/day (maximum adult dose: 600 mg every 8 h) |
| Hospital treatment | Cefuroxime IV 150−200 mg/kg/day(adult dose: 750−1,500 mg every 8−12 h) | Levofloxacin:< 5 years: 20 mg/kg/day in 2 IV or PO doses≥ 5 years: 10 mg/kg/day in 1 IV or PO dose |
IV: intravenous; PO: oral route.
The severity criteria that appear in the different guidelines have been developed by consensus among the authors and are not validated for CAP in children.124 Several comorbidities, such as malnutrition, congenital heart disease, Down syndrome, cerebral palsy, or acquired immunodeficiency syndrome, have been associated with increased mortality from pneumonia.124
Other factors that could predict greater severity are: hypoxemia, age less than 3−6 months, multilobar alveolar infiltrates and large pleural effusions, major respiratory distress, or complication due to sepsis.1,124
Admission to the PICU is necessary when pneumonia causes significant respiratory distress or is complicated by sepsis. Table 7 shows the criteria recommended in this document for admission to the hospital ward and the PICU.1,2,120
Therapeutic failureResponse to the treatment of pneumonia is considered poor when respiratory failure develops, or tachypnea, fever or compromised general condition persists 48−72 h after starting.2,125
Parapneumonic pleural effusionParapneumonic pleural effusion (PPE) is defined as the association of pleural effusion and pneumonia, and occurs in approximately 1/150 (0.6%) of CAP cases.126 Pulmonary empyema is the presence of pus in the pleural space. Some authors extend the concept of empyema to the identification of bacteria in the pleural fluid Gram stain, or to the presence of biochemical alterations in pleural fluid indicative of complicated effusion.127 Light criteria have long been used in adults and children to indicate when a parapneumonic effusion should be drained (pH ≤ 7.2, glucose < 40 mg/dl, lactate dehydrogenase > 1000 IU/l).128 However, these criteria are based on studies in adults and have not been validated in children, so they are not currently recommended for use as unique criteria on which to base the decision to place a chest tube in the pediatric population129,130 (strong recommendation, weak evidence).
The main utility of pleural fluid analysis in children is the identification of the causative agent, which helps to select the appropriate antibiotic.1,118 The white blood cell and differential count are also useful in the differential diagnosis of bacterial or tuberculous effusions. In our setting, approximately 2%-2.5% of patients presenting with fever and parapneumonic effusion will have a diagnosis of tuberculosis.131,132
Pleural drainageIt is recommended that pleural drainage techniques be performed by expert personnel, with the patient under sedation or general anesthesia (obligatory in young children or in cases of respiratory distress), in a hospital treatment room adequately equipped for this purpose.132,133 Most studies132,134 conclude that percutaneous insertion of small catheters (8.5–14 Fr) is preferable to large surgical chest tubes (strong evidence, moderate recommendation). These catheters are as effective as the larger type, better tolerated, produce less discomfort, and may even reduce the hospital length of stay.134,135
The best puncture site is usually between the 5th and 7th intercostal space, at the level of the mid-posterior axillary line120 (strong recommendation, high evidence). If a more posterior insertion site is used, it should be noted that in this area, the arteries run in the middle of the intercostal spaces, thus increasing the risk of traumatic insertion.129 Puncture should not be performed if the effusion is subpulmonary.
It is advisable that the puncture be guided by ultrasound, either by marking the puncture point or preferably by real-time ultrasound,134 allowing continuous monitoring during the catheter placement procedure. Seldinger's technique for percutaneous insertion is usually preferable, as it appears to be safer than the introduction of a trocar, but it should be noted that the rigid dilator can also cause injury,133 and some authors prefer to use a trocar that can be better visualized with ultrasound.134
In order to check the position of the tube and rule out pneumothorax, it is advisable to perform a chest X-ray after placement. The tube is connected to a one-way drainage system, which must always be kept below the height of the patient's chest.120 Fluid extraction is best achieved by suction with a water seal, at a pressure of 5−10 cm. Drainage should be suspended for 1 h when an amount of 10 mL/kg is collected.120 In older children or adolescents, no more than 1.5 L should be drained at a time, or up to about 500 mL/h with slow drainage.120 The tube is usually removed when the fluid yield is minimal (< 40−60 ml/24 h)132 or < 1 mL/kg/day the previous 12 h; it does not have to be clamped first.
FibrinolyticsIntrapleural fibrinolytics improve the elimination of pleural fluid, especially in the presence of loculation and complex septation on ultrasound.136 Loculations and septations correlate moderately with the presence of pus, but anechoic effusions may also contain frank pus.134
The results of a meta-analysis that included clinical trials in children and adults comparing drainage alone with drainage combined with fibrinolytics suggest that fibrinolytic therapy decreases hospital stay and that the use of urokinase (strong recommendation, high evidence), but not streptokinase or tissue plasminogen activator (tPA), it is more effective than drainage alone in reducing the need for reintervention, without increasing the incidence of serious side effects.137
Streptokinase is associated with an increased risk for adverse effects such as fever or allergic reactions,138 while tPA is more expensive. In an adult clinical trial, the use of tPA combined with dornase alfa (DNase) decreased hospital stay and the frequency of surgery, while the use of either tPA or DNase alone was ineffective.139 A clinical trial in children aged 6 months to 18 years, in which DNase was added to the intrapleural tPA, failed to show additional benefit for the combination of DNase plus fibrinolytic in length of hospital stay or other outcomes compared with tPA plus placebo.136
No studies have been published comparing the effectiveness of different fibrinolytics in the treatment of empyema in children.140 This consensus document recommends the use of urokinase given the greater experience in children,132,137 at the following dosage120,132:
- -
Children < 1 year: 10,000 units in 10 mL of 0.9% saline.
- -
Children ≥ 1 year: 40,000 units in 40 mL of 0.9% saline.
It is administered twice a day, for 3 days, through the pleural drainage tube, which is then clamped for 4 h, followed by 8 h of aspiration with a negative pressure of 10–20 cmH2O. Additional doses may be used if response is incomplete after these 6 doses.129
The administration of fibrinolytic agents may be painful and should be combined with adequate analgesia. Other side effects may include: mild bleeding and, rarely, immediate hypersensitivity reactions (with streptokinase). They are contraindicated in the case of bronchopleural fistula or when bubbling is seen in the drainage tubes, which would suggest an air leak that could cause a tension pneumothorax if the tube is clamped.120
In any case, if the child has signs of clinical deterioration, such as increased respiratory distress, the drainage tube should be unclamped.120
Video-assisted thoracoscopyVideo-assisted thoracoscopy (VATS) is useful for staging the effusion, rupturing septae, draining fibrinopurulent material, reducing the bacterial load in the early stages, and placing the drainage tube in the correct position.120 It is also useful for visualizing the appearance of the underlying lung, its expansion capacity, and the location of possible bronchopleural fistulas.120 The complication rate, including air leak or persistent pneumothorax, pneumatocele, or bleeding, is low (6%-7%).141
VATS is used in some centers as initial treatment of moderate or large PPEs,142 since, compared to drainage alone, it reduces the duration of fever, which usually resolves 24−72 h after the procedure, and shortens hospital stay,126,143 although it is more commonly used in the treatment of cases in which drainage with fibrinolytics has failed (moderate recommendation, moderate evidence) and in the treatment of complications, such as bronchopleural fistulas.
Pleural effusion management algorithmThere is no universal consensus on the treatment of parapneumonic effusion, since in many respects the evidence is weak, partial, or non-existent.126 In our setting, mortality is minimal in children and the long-term prognosis is excellent. The different therapeutic approaches are proposed to optimize health resources, including shortening hospital stay.
For the management of effusions, it is useful to classify them as144:
- -
Small: < 10 mm on lateral X-ray, or < 1/4 of hemitorax on anterior-posterior (AP) X-ray.
- -
Moderate: > 1/4 and ≤ 1/2 on AP X-ray.
- -
Large: > 1/2 on AP X-ray.
Small effusions respond well to antibiotic treatment without the need for drainage. If significant respiratory compromise or uncontrolled sepsis occurs despite antibiotic treatment, interventional therapy is indicated.140,144 Most guidelines1,120,129 recommend pleural drainage associated with fibrinolytics or VATS in all cases of moderate and large effusion, since the duration of treatment and hospital stay is considerably longer in cases treated with antibiotic alone or with drainage alone120,129(strong recommendation, high evidence). However, in recent series,131,144,145 a percentage of children with moderate (50%) or large (25%-30%) effusion showed good resolution with antibiotic treatment alone, without the need for drainage and without prolonging hospital stay,144 suggesting that there may be a subgroup of children with moderate or large effusions in whom interventional treatment may not be necessary. To our knowledge, these approaches − antibiotic-only treatment, initial antibiotic treatment and intervention at 2−3 days in case of lack of response, or initial interventional treatment − have not been compared in any clinical trial.
In a retrospective study, delays of 1−2 day in chest drainage placement were associated with lower mortality than delays of more than 7 days.145
There is good evidence from clinical trials in children to support interventional treatment where this approach is needed or selected. The largest study was a multicenter study conducted in Spain, with 50 patients in the VATS group and 53 in the fibrinolytic group,132 while another 2 studies have been performed, 1 in the United Kingdom (UK) and 1 in the US, with a maximum number of 30 children per arm.146,147 None of these 3 studies revealed any differences between the 2 groups in terms of hospital stay or complications (strong recommendation, high evidence). The cost of fibrinolysis was lower than that of VATS, 35% less in the US study and 20% less in the UK. Therefore, given the greater simplicity of fibrinolytic administration that does not require surgical intervention, this approach is proposed as the first option before the use of VATS,1,120,143 which should be reserved for non-responders.
A meta-analysis140 revealed no differences in perioperative complications between the use of fibrinolytics and VATS. The need for reintervention and the duration of postoperative hospital stay were both lower in the VATS group (–0.7 days). However, this meta-analysis includes a clinical trial that compared VATS with drainage alone, and 4 observational studies, which, along with the small differences in hospital stay, does not warrant modifying the previous recommendation.140
A recent Spanish multicenter study (Spanish Multicenter Study on the Use of Dexamethasone in Parapneumonic Pleural Effusion [CORTEEC])148 included 60 children who were randomized to receive antibiotic treatment at baseline, combined with either dexamethasone (0.25 mg/kg/6 h IV) or placebo for 2 days. In children who received dexamethasone, the time to recovery was shorter (median 2.8 days), but the risk of hyperglycemia was higher. In just over 90% of cases, effusions were small (<1/3 hemitorax), so the results cannot be extrapolated to patients with larger effusions.
In summary, the choice of treatment for parapneumonic effusions in hospitalized children appears to depend largely on the preferences of physicians based on their experience and the resources available.143 In the US, the use of VATS peaked about 10 years ago, and this technique is gradually being replaced by pleural drainage and fibrinolytics.143
In a survey conducted in Spain on the best approach to initial treatment in children with parapneumonic effusion or empyema,149 44% respondents preferred chest drainage with fibrinolytics; 18%, antibiotics only; 17%, simple drainage; and 17%, VATS. Overall, 54% of the centers said they followed the guidelines published in Spain. Another survey conducted in Central European countries also revealed a complete lack of agreement on the therapeutic approach to parapneumonic effusions in children.138 There is also a lack of consensus on the use of diagnostic thoracentesis in cases where no chest tube is placed; in the European survey, it was only used in 3% of cases138 and in the Spanish survey it was used in 46%.149
Multicenter clinical trials are thus needed to provide more evidence for the management of parapneumonic effusions in children and to develop evidence-based algorithms.137
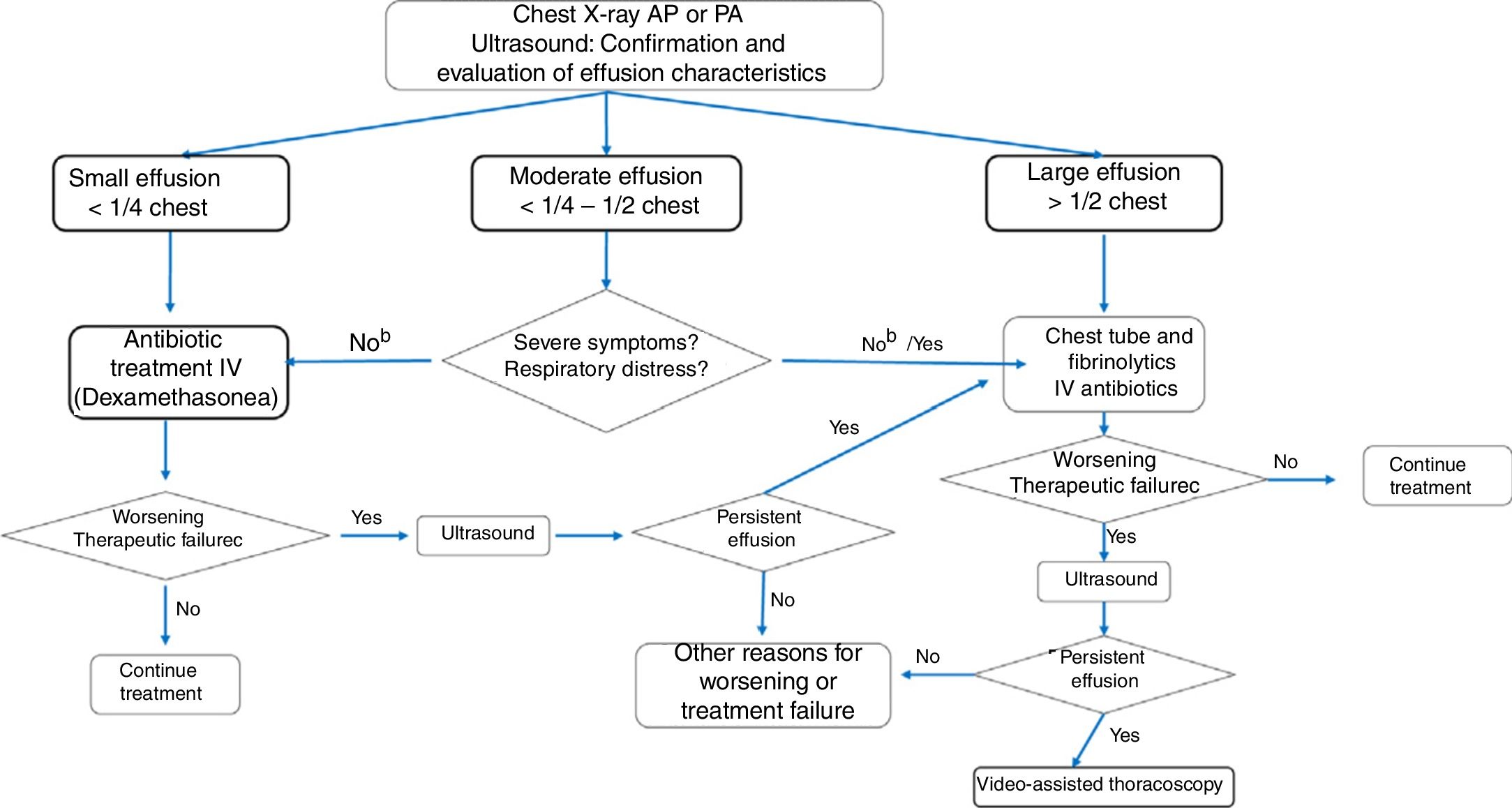
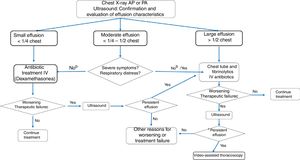
Fig. 2 shows the algorithm recommended by this consensus document, following the best available evidence and expert agreement.
Algorithm for significant parapneumonic pleural effusion.
a Dexamethasone 0.25 mg/kg/6 h for 2 days at the beginning of treatment may reduce recovery time in small effusions. b For moderate parapneumonic effusion without severe symptoms, the options are to administer antibiotic treatment only, to which approximately 50% of patients will respond, or to place a chest tube and administer intrapleural fibrinolytics. If pleural drainage is not selected, diagnostic thoracentesis may be an option. c Worsening or treatment failure: development of respiratory failure or persistent tachypnea, fever, or deterioration of general condition within 48−72 hours of starting antibiotic therapy (duration of fever should not be the only criterion in cases of antibiotic treatment without drainage). dOther causes of poor progress may include the presence of necrotizing pneumonia or the development of a pulmonary abscess.
Vaccination against certain microorganisms has been shown to have an impact on the incidence and mortality of CAP. The etiologic agents for which vaccines are available are S. pneumoniae, H. influenzae type b and influenza virus.150
The inclusion of pneumococcal conjugated vaccines, both PCV13 and PCV10, in the official pediatric immunization schedule in more than 150 countries worldwide has shown a moderate reduction in cases and hospitalization for CAP in children, especially in children under 5 years of age150–158 (strong recommendation, high evidence), although the data on the impact of group immunity on the incidence of CAP in adults, including unvaccinated individuals over 65 years of age, is conflictin.153–156 In Spain, data have not been published since the introduction of routine vaccination with PCV13 in 2016, but experience in the Community of Madrid has shown that systematic vaccination of children reduces the incidence of CAP, including complicated forms, caused by serotypes contained in PCV13.158 In countries such as France, which has greater experience with routine PCV13 vaccination, pneumococcal pneumonia in children has reduced significantly, with S. pyogenes currently being the most frequent cause of parapneumonic pleural empyema (45%).159
The AEP Vaccine Advisory Committee recommends systematic H. influenzae type b and PCV13 pneumococcal vaccination in all children under 5 years of age.160–163 Moreover, children in risk groups should receive the influenza vaccine after 6 months of age and the 23-valent unconjugated vaccine after 24 months of age.160–163
Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and Spanish Society of Pediatric Pulmonology (SENP)
Please cite this article as: Andrés-Martín A, Montaner AE, Mulet JF, García MLG, Murua JK, Moreno-Pérez D, et al. Documento de consenso sobre la neumonía adquirida en la comunidad en los niños. SENP-SEPAR-SEIP. Arch Bronconeumol. 2019. https://doi.org/10.1016/j.arbres.2020.03.025
- Home
- All contents
- Publish your article
- About the journal
- Metrics