The term phenotype in the field of COPD is defined as “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes”. Among all phenotypes described, there are three that are associated with prognosis and especially with a different response to currently available therapies. The phenotypes are: the exacerbator, the overlap COPD-asthma and the emphysema-hyperinflation.
The exacerbator is characterized by the presence of, at least, two exacerbations the previous year, and on top of long-acting bronchodilators, may require the use of anti-inflammatory drugs. The overlap phenotype presents symptoms of increased variability of airflow and incompletely reversible airflow obstruction. Due to the underlying inflammatory profile, is used to have a good therapeutic response to inhaled corticosteroids in addition to bronchodilators. Lastly, the emphysema phenotype presents a poor therapeutic response to the existing anti-inflammatory drugs, and long-acting bronchodilators together with rehabilitation are the treatments of choice.
Identifying the peculiarities of the different phenotypes of COPD will allow us to implement a more personalized treatment, in which the characteristics of the patients, together with their severity will be key to choose the best treatment option.
El término fenotipo aplicado a la EPOC se define como “aquellos atributos de la enfermedad que solos o combinados describen las diferencias entre individuos con EPOC en relación a parámetros que tienen significado clínico”. De entre todos los descritos, existen tres que se asocian con factores pronósticos y sobretodo con distinta respuesta a los tratamientos disponibles en la actualidad. Estos fenotipos son: el agudizador, el mixto EPOC-asma y el enfisema-hiperinsuflado.
El agudizador se caracteriza por la presencia de la menos dos agudizaciones el año previo y además del tratamiento con broncodilatadores de larga duración puede requerir la utilización de fármacos antiinflamatorios. El fenotipo mixto presenta una obstrucción no completamente reversible al flujo aéreo acompañada de una reversibilidad aumentada de la obstrucción. Por su perfil inflamatorio subyacente suele presentar una buena respuesta terapéutica a los corticosteroides inhalados unidos a los broncodilatadores. Por último el fenotipo enfisema presenta una pobre respuesta a los fármacos antiinflamatorios de que disponemos en la actualidad y los broncodilatadores de larga duración, junto a la rehabilitación son la base de su tratamiento.
El reconocimiento de las peculiaridades de los distintos fenotipos de la EPOC nos debe permitir guiar un tratamiento más personalizado, en el que las características del paciente se sumen a su gravedad para dirigir la terapia.
In recent years, the term “phenotype” has been used to refer to clinical types of patients with chronic obstructive pulmonary disease (COPD).1,2 This has been motivated by the boom in studies that propose to identify genetic determinants for developing the disease in its different manifestations. An international group of experts has defined COPD phenotype as “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to treatment, speed of progression of the disease or death)”.1 Therefore, the phenotype should be able to classify the patients into subgroups with a prognostic value that allow for determining the best therapy in order to achieve better clinical results.1–3
According to the opinion of the majority, the term “COPD phenotype” is reserved for the different clinical types that have therapeutic impact and are identified in COPD patients. In recent years, several researchers have attempted to quantify the different “faces” or phenotypes of COPD in the so-called non-proportional Venn diagram of COPD,4 that demonstrates the great confusion existing among the several etiopathogenic, clinical and morphologic types of this syndrome that we call COPD, although some have postulated that it should be defined as a group of orphan diseases.5
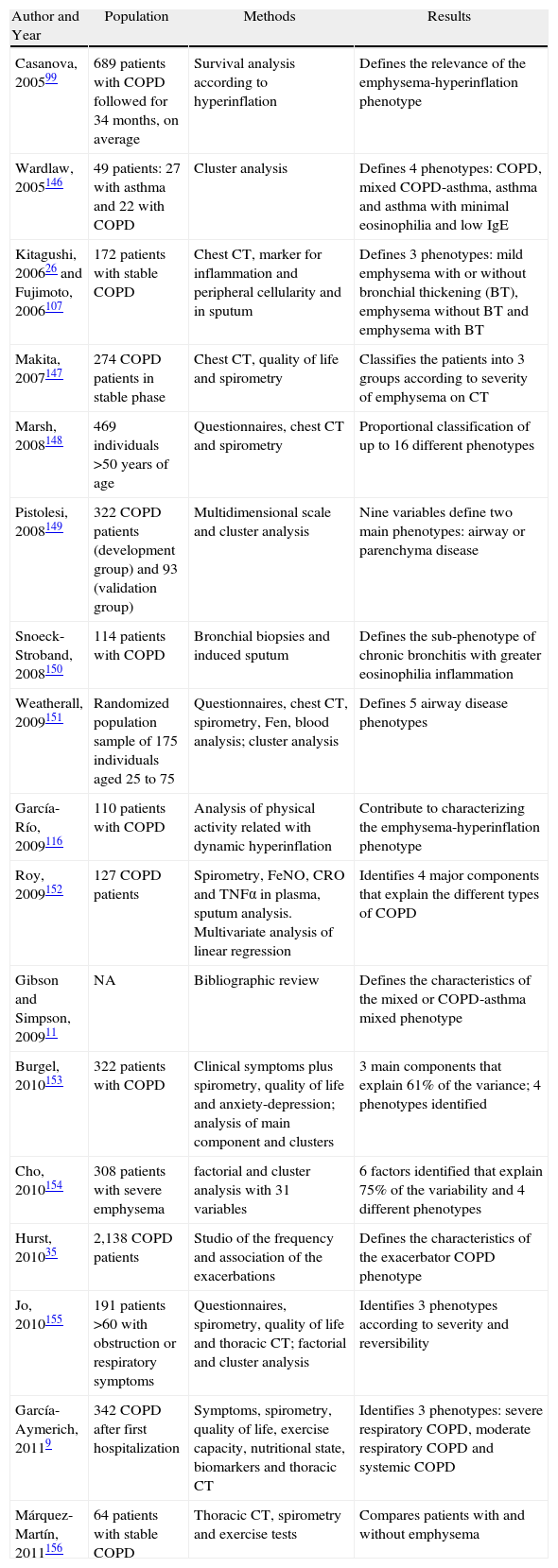
Phenotypes of Clinical Interest in COPDWe should accept that there needs to be a mid-point between the excessive simplification of the term COPD (a definition that encompasses the entire spectrum of patients with non-completely reversible airflow obstruction) and the complexity of considering each patient individually as an orphan disease. This “happy medium” entails the identification and description of some phenotypes that are not only of biological and epidemiological interest but also of prognostic and above all therapeutic interest. Table 1 shows some studies that have identified several clinical phenotypes in COPD. These studies are based on heterogeneous populations, using diverse methodologies to analyze different variables, but they all reach similar conclusions: it is possible to distinguish between different patterns of clinical expression in COPD—the so-called phenotypes. The majority distinguish between 3 and 5 phenotypes based on a series of factors that are enumerated in Table 2.
Studies That Have Identified Phenotypes in COPD.
| Author and Year | Population | Methods | Results |
| Casanova, 200599 | 689 patients with COPD followed for 34 months, on average | Survival analysis according to hyperinflation | Defines the relevance of the emphysema-hyperinflation phenotype |
| Wardlaw, 2005146 | 49 patients: 27 with asthma and 22 with COPD | Cluster analysis | Defines 4 phenotypes: COPD, mixed COPD-asthma, asthma and asthma with minimal eosinophilia and low IgE |
| Kitagushi, 200626 and Fujimoto, 2006107 | 172 patients with stable COPD | Chest CT, marker for inflammation and peripheral cellularity and in sputum | Defines 3 phenotypes: mild emphysema with or without bronchial thickening (BT), emphysema without BT and emphysema with BT |
| Makita, 2007147 | 274 COPD patients in stable phase | Chest CT, quality of life and spirometry | Classifies the patients into 3 groups according to severity of emphysema on CT |
| Marsh, 2008148 | 469 individuals >50 years of age | Questionnaires, chest CT and spirometry | Proportional classification of up to 16 different phenotypes |
| Pistolesi, 2008149 | 322 COPD patients (development group) and 93 (validation group) | Multidimensional scale and cluster analysis | Nine variables define two main phenotypes: airway or parenchyma disease |
| Snoeck-Stroband, 2008150 | 114 patients with COPD | Bronchial biopsies and induced sputum | Defines the sub-phenotype of chronic bronchitis with greater eosinophilia inflammation |
| Weatherall, 2009151 | Randomized population sample of 175 individuals aged 25 to 75 | Questionnaires, chest CT, spirometry, Fen, blood analysis; cluster analysis | Defines 5 airway disease phenotypes |
| García-Río, 2009116 | 110 patients with COPD | Analysis of physical activity related with dynamic hyperinflation | Contribute to characterizing the emphysema-hyperinflation phenotype |
| Roy, 2009152 | 127 COPD patients | Spirometry, FeNO, CRO and TNFα in plasma, sputum analysis. Multivariate analysis of linear regression | Identifies 4 major components that explain the different types of COPD |
| Gibson and Simpson, 200911 | NA | Bibliographic review | Defines the characteristics of the mixed or COPD-asthma mixed phenotype |
| Burgel, 2010153 | 322 patients with COPD | Clinical symptoms plus spirometry, quality of life and anxiety-depression; analysis of main component and clusters | 3 main components that explain 61% of the variance; 4 phenotypes identified |
| Cho, 2010154 | 308 patients with severe emphysema | factorial and cluster analysis with 31 variables | 6 factors identified that explain 75% of the variability and 4 different phenotypes |
| Hurst, 201035 | 2,138 COPD patients | Studio of the frequency and association of the exacerbations | Defines the characteristics of the exacerbator COPD phenotype |
| Jo, 2010155 | 191 patients >60 with obstruction or respiratory symptoms | Questionnaires, spirometry, quality of life and thoracic CT; factorial and cluster analysis | Identifies 3 phenotypes according to severity and reversibility |
| García-Aymerich, 20119 | 342 COPD after first hospitalization | Symptoms, spirometry, quality of life, exercise capacity, nutritional state, biomarkers and thoracic CT | Identifies 3 phenotypes: severe respiratory COPD, moderate respiratory COPD and systemic COPD |
| Márquez-Martín, 2011156 | 64 patients with stable COPD | Thoracic CT, spirometry and exercise tests | Compares patients with and without emphysema |
IgE: immunoglobulin E; CT: computed tomography; FeNO: exhaled fraction of nitric acid; CRP: C-reactive protein; TNFα: tumor necrosis factor-alpha.
Factors or Variables Identified as Significant for the Classification of COPD Patients.
| Author and Year | Factors |
| Casanova, 200599 | IC/TLC |
| Wardlaw, 2005146 | Eosinophilia in sputumReversibility of the obstructionIgE |
| Kitagushi, 200626 and Fujimoto, 2006107 | Quantification of CT for parenchyma and airwayReversibility in percentageEosinophilia in sputum and peripheralRespiratory symptoms |
| Makita, 2007147 | Emphysema by CTBMIQuality of life |
| Pistolesi, 2008149 | Sputum volume and appearanceQuantification of CT for parenchyma and airwayPulmonary soundsFEV1/FVCAir trapping |
| Snoeck-Stroband, 2008150 | Eosinophilia in sputum in chronic bronchitis |
| Weatherall, 2009151 | AgeFEV1 and FEV1/FVCReversibility, percentageKco, percentageFRC, percentageIgEFeNOSmoking, pack-years |
| García-Río, 2009116 | Physical activity related with dynamic hyperinflation |
| Roy, 2009152 | Neutrophilia in sputum, IL-8 and TNFαEosinophilia in sputum and FeNOReversibility to bronchodilator, FEV1 and ICCRP |
| Gibson and Simpson, 200911 | SymptomsFEV1Bronchial hyperreactivityEosinophilia in sputum |
| Burgel, 2010153 | FEV1AgeSymptomsComorbidity |
| Cho, 2010154 | FEV1 post-bronchodilatorBronchodilator response, percentageQuantitative measurement of emphysema and bronchial wall on CT |
| Hurst, 201035 | FEV1Frequency of exacerbations in the pastGastroesophageal refluxQuality of life |
| Jo, 2010155 | AgeReversibility, percentagePost-bronchodilator FEV1 |
| García-Aymerich, 20119 | Severity of respiratory symptomsComorbidity and systemic inflammation |
| Márquez-Martín, 2011156 | Emphysema by CTPeripheral muscle strengthExercise capacityBMI |
CT: computed tomography; FEV1: forced expiratory volume in one second; BMI: body mass index; IC/TLC: inspiratory capacity/total lung capacity; IgE: immunoglobulin E; VC: vital capacity; FVC: forced vital capacity; Kco: carbon monoxide transfer coefficient; FRC: functional residual capacity; FeNO: exhaled fraction of nitric acid; Il: interleukin; TNFα: tumor necrosis factor alpha; CRP: C-reactive protein.
After analyzing these studies, we can conclude that there is evidence to define at least three different phenotypes with clinical, prognostic and therapeutic repercussions: (1) “overlap” or mixed COPD-asthma; (2) exacerbator; and (3) emphysema-hyperinflation.
Other possible phenotypes have been defined, but these have had little clinical transcendence. Thus, the so-called “fast decliner” would be a patient who suffers a loss of lung function, expressed by FEV1, that is faster than average.6 The practical problem is that it is impossible to identify this phenotype without a strict follow-up of the lung function for at least 2 years; on the other hand, no specific treatment has been identified for this type of patients. Another possible phenotype would be chronic bronchitis, defined as cough and expectoration for at least 3 months of the year for 2 consecutive years.7 This phenotype is usually associated with airway disease, which can be visualized with high-resolution computed tomography (HRCT).8 Nevertheless, chronic bronchitis can accompany any of the three phenotypes indicated beforehand: “mixed”, exacerbator and emphysema. We therefore prefer to describe it as a modifying factor in any of the 3 main phenotypes. A systemic phenotype has also been defined in patients who present obesity, cardiovascular disease, diabetes or systemic inflammation.9 It is true that these patients present a different prognosis, but we cannot call “systemic” COPD a phenotype as it does not meet the anterior definition, as the systemic manifestations (or comorbidities) have not been shown to be a manifestation of the COPD itself. The systemic manifestations or comorbidities are very important, but they should be considered apart from the phenotype.
Last of all, one special phenotype is emphysema due to alpha-1-antitrypsin deficiency, which is characterized by predominantly basal emphysema that appears at early stages in life, especially in smokers, and it has a genetic base.10 Due to its limited prevalence, we prefer to consider it apart from the general classification.
Mixed COPD-Asthma PhenotypeWhen a patient presents characteristics of more than one obstructive airway disease, we say that he/she has an overlap or mixed syndrome. The guidelines of the American Thoracic Society (ATS) from 1995 defined obstructive disease and identified 11 different syndromes, 6 of which were overlap syndromes.7 A study that used data from a very extensive population observed that 19% of patients with airflow obstruction had more than one disease present.4 The most representative and frequent diseases or processes within these subgroups were chronic airflow obstruction and asthma. Therefore, it should not seem strange that there are a good number of patients who share characteristics that are attributed to COPD and asthma. This population is of special interest as it usually sidelined in clinical pharmaceutical trials. Asthma studies tend to exclude smokers, and COPD studies usually exclude individuals with a previous history of asthma. Some even exclude those individuals with a positive bronchodilator test.
Definition of the Mixed Phenotype (COPD-Asthma)The mixed phenotype in COPD is defined as an airflow obstruction that is not completely reversible, accompanied by symptoms or signs of increased obstruction reversibility.11
Justification of the Mixed PhenotypePathogenesis and PrevalenceWithin the spectrum of chronic airway obstruction, there are individuals with asthma who smoke, asthmatics who develop airflow obstruction that is not completely reversible and non-smokers who develop chronic airflow obstruction. Smokers with asthma have features that mimic COPD, with less response to corticosteroids, a lower frequency of eosinophilic inflammation and a greater probability of neutrophilia in the airways.12,13 On the other hand, there are epidemiological studies about the incidence of COPD which demonstrate that young asthmatics who develop COPD have a disease with different characteristics than the non-asthmatics who also develop COPD. In the first case, allergic rhinitis is more frequent, along with nonspecific bronchial hyperreactivity and the presence of wheezing, and plasma IgE concentrations are higher14; all of which indicate that it is a mixed asthma-COPD syndrome.
The prevalence of the mixed phenotype is unknown, but there are different estimations of its importance in the context of COPD. One initial study that was small in size estimated that 25% of COPD patients with COPD have significant reversibility and presented clinical response to inhaled corticosteroids (IC).15 Soriano et al.4 estimated that approximately 23% of COPD patients between the ages of 50 and 59 could have a mixed phenotype, which increased with age up to 52% between the ages of 70 and 79.4 Other studies have quantified the prevalence of the mixed phenotype (identified by eosinophilia in sputum) in patients with COPD at 38%, directly associated with the therapeutic response to IC.16 If we use the bronchodilator test as a reference, 31.5% of the patients identified with COPD in the EPI-SCAN epidemiological study had a positive test.17 Based on these results, we can conclude that, together, between 20% and 40% of COPD patients can be carriers of a mixed phenotype.
Differential TreatmentThe clinical justification for the mixed phenotype lies in its demonstrated sensitivity to the anti-inflammatory action of IC. The basis that explains the response to corticosteroids in COPD patients with greater reversibility lies within the etiopathogeny of the disease. Papi et al.18 demonstrated that reversible patients, even those who were only partially reversible (increase in FEV1>200ml, but <12%) had greater eosinophilic bronchial inflammation compared with the irreversible ones, in whom neutrophilic inflammation predominated. In fact, several studies have used the greater airflow reversibility,4,19–21 a high concentration of eosinophils in spontaneous or induced sputum16,22 or a greater concentration of exhaled NO23–25 as markers of the response to IC in COPD, both at the lung function level15,16,19–23,25 as well as the improvement of the symptoms.22,24,25 A more recent study classified a small group of patients into 3 different phenotypes, according to the findings from chest computed tomography (CT).26 The authors demonstrated a relationship between the response to the bronchodilator test, the response to treatment with IC and the concentration of eosinophils in sputum in each of the 3 phenotypes.26 There is even a randomized clinical assay that compared the treatment with IC in patients with COPD, directed in accordance with the current guidelines or according to the concentration of eosinophils in induced sputum. The results demonstrated a significant reduction of the exacerbations during one year in those patients who took IC based on their eosinophilic inflammation profile.27 All these results justify a personalized focus in IC treatment based on the clinical, functional and inflammatory characteristics of COPD patients.28,29
With regards to the combined treatment of long-acting beta-2 adrenergics (LABA) and IC, it is important to remember that the first published results demonstrated that the combined treatment with fluticasone/salmeterol (FSC) was effective for achieving an important bronchodilation in COPD patients, but it should be remembered that half of the patients studied had a positive bronchodilator test at the beginning of the study.30 When the results were analyzed separately, the reversible patients achieved a maximum bronchodilator effect of 319ml FEV1, while the irreversible patients reached 195ml.30
The limited reduction in mortality observed in the Towards a Revolution in COPD Health (TORCH) study with FSC can be explained, at least in part, by the patient selection. One of the inclusion criteria was to have a negative bronchodilator test, which is reflected in a mean reversibility of the participants of only 3.7%.31 Therefore, TORCH explains the long-term effect of the FSC combination in those patients who are less susceptible of being responders to IC. Contrarily, a more recent study has compared FSC with salmeterol in treating patients with severe COPD (FEV1 <50%).32 Its results showed a significant reduction (35%) during one year in the rate of moderate or severe exacerbations with FSC compared with salmeterol alone. This study did not take into account the reversibility of FEV1 among its inclusion criteria, and in fact the mean reversibility of its patients was 7%, almost the double of TORCH, and the result was a spectacular reduction in the frequency of exacerbations by adding fluticasone to the treatment with salmeterol. In the same direction, in a recent study it was demonstrated that treatment with FSC at a dosage of 250/50 every 12h produced an increase in the area under the curve 6h after FEV1 that was more than double in reversible patients (1.98lh in week 8) than in irreversible ones (0.74lh). This provides more evidence of the different response to IC or combined treatment depending on the response to the bronchodilator test.20 It has also been recently shown that there is a direct and significant correlation between the response to the bronchodilator test with salbutamol in COPD and the improvement in lung function after 3 months of treatment with LABA+IC.21 In contrast, patients with a defined phenotype such as emphysema-dominant did not present any improvement in lung function with the same treatment.21 It should be expected that these findings described in the studies carried out with salmeterol or with SAL/FLU may be extrapolatable to other IC or LABA/IC combinations.
In short, the conclusions that we may draw from the existing studies are: (a) the patients with mixed phenotype, who present certain characteristics (sputum or peripheral eosinophilia, history of asthma and/or atopy, frequent exacerbations, very positive bronchodilator test or wheezing as a guiding sign) are susceptible for presenting a good response to IC, whatever the lung function; (b) COPD patients who do not present the former characteristics will obtain marginal clinical benefits with the use of IC added to long-acting bronchodilators.
Diagnosis of the Mixed Phenotype (COPD-Asthma)In order to be able to identify the mixed phenotype, the clinical history will serve as a guide, showing history of asthma and atopy in childhood and youth, less intense smoking exposure, frequency of exacerbations and key symptoms such as wheezing, among others. But in order to characterize a patient with COPD as mixed, it is also necessary to carry out a series of tests. Spirometry, in addition to diagnosing the disease, provides a measurement of its severity, and the magnitude of the reversibility in the bronchodilator test will guide us towards the possible diagnosis as mixed. The blood work-up reveals if there is eosinophilia, and in an ideal situation the cytologic analysis of the sputum may be able to indicate the intensity of the eosinophilic inflammation, while a high concentration of exhaled nitric oxide could also help to identify patients with mixed COPD-asthma phenotype.
The utility of the bronchodilator test in determining the response to IC has been brought into doubt after the results reported by Calverley et al.33 In the screening phase of the ISOLDE study, they analyzed a total of 660 patients. By carrying out 3 bronchodilator tests in a 2-month period, they observed that a significant number of patients could be either positive or negative for the different tests; therefore, they concluded that it was not adequate to classify the patients as reversible or irreversible. Nevertheless, it must be kept in mind that they excluded the patients who on the first test had a reversibility of FEV1 >10%, and that the protocol of the test was different on the 3 different occasions that it was performed. This study does not invalidate the utility of the bronchodilator test, but it reminds us that response is a continuous variable and that it is more reliable to interpret the magnitude of the response in each case than to classify a patient as either reversible or irreversible according to an arbitrary cut-point.
In short, the diagnosis of the mixed phenotype will be established by the presence of a combination of the following factors: history of asthma and/or atopy, reversibility in the bronchodilator test, notable eosinophilia in respiratory and/or peripheral secretions, high IgE, positive prick test to pneumoallergens and high concentrations of exhaled NO.
Exacerbator PhenotypeThe clinical course of COPD is frequently punctuated by episodes of clinical instability, which we refer to as exacerbations. It is estimated that COPD patients suffer between 1 and 4 exacerbations per year34; however, their appearance does not follow a normal distribution. Some patients do not suffer any exacerbations, while others experience them repeatedly. In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study, which is a prospective observational study including 2138 patients with moderate-severe COPD followed for 3 years,35 23% of the patients did not have any exacerbations, while 12% of the cases had 2 or more exacerbations per year over the course of the 3-year study period. The exacerbators maintained a notable stability over time to the point that somewhat more than 60% of the patients with 2 or more exacerbations in the first year also had frequent exacerbations in the second year of follow-up, and out of these more than 70% continued having repeated decompensation in the third year. Given this stability over time, it has been suggested that these patients could present individual susceptibility for suffering frequent decompensations.36,37 This fact, and also the fact that we are faced with a patient group with a high risk for morbidity and mortality38–45 whose treatment could be differentiated, constitute the rationale for defining the “exacerbator” phenotype. The cut-point of the number of exacerbations for considering a patient an exacerbator has varied over time, but currently exacerbators are considered those patients who present 2 or more exacerbations per year.35
Definition of “Exacerbator”“Exacerbators” are defined as those COPD patients who present with 2 or more exacerbations per year. These exacerbations should be separated by at least 4 weeks after the end of treatment of the previous exacerbation or 6 weeks after the onset of the exacerbation in cases that have received no treatment. This is in order to be able to differentiate between the new event and previous therapeutic failure.36
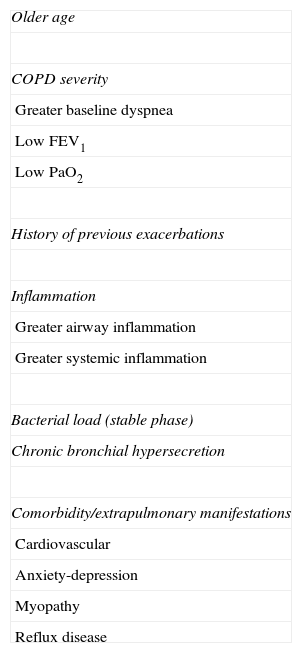
Justification of the Exacerbator PhenotypeIndividual Susceptibility for Suffering Frequent ExacerbationsTable 3 compiles the main risk factors linked to the presence of repeated exacerbations.38,40,41,43–61 The severity of the airflow limitation is without doubt one of the most well-known factors.38,40,46,62,63 However, the relationship between FEV1 and number of exacerbations is not linear, and in fact close to 40% of the severe or very severe patients do not present exacerbations, while more than 20% of the moderate patients frequently present them.38,46 This suggests the existence of other conditioning factors. Of all of them, the history of previous exacerbations is the most frequent factor referenced in the literature,38,40,43,49–53 which emphasizes the existence of a certain individual susceptibility that may either be hereditary or acquired.
Risk Factors Associated With Repeated Exacerbations.
| Older age |
| COPD severity |
| Greater baseline dyspnea |
| Low FEV1 |
| Low PaO2 |
| History of previous exacerbations |
| Inflammation |
| Greater airway inflammation |
| Greater systemic inflammation |
| Bacterial load (stable phase) |
| Chronic bronchial hypersecretion |
| Comorbidity/extrapulmonary manifestations |
| Cardiovascular |
| Anxiety-depression |
| Myopathy |
| Reflux disease |
The presence of cough and chronic expectoration is associated with a greater risk for repeated exacerbations.57 Foreman et al.58 found that the odds ratio (OR) for exacerbation was 3.7 for patients with chronic expectoration, much higher than the risk observed related to accumulated tobacco consumption (OR: 1.01, for each pack-year) or post-bronchodilator FEV1% (OR: 0.98). Similar results have also been reported by Miravitlles et al.,34 who observed a significant association between chronic mucus hypersecretion and the presence of two or more exacerbations in the previous year (OR, 1.54). Likewise, Burgel et al.49 verified that among the patients with frequent exacerbations, 55% had associated chronic expectoration and cough, compared with 22% of cases without bronchial hypersecretion (P<.001), with a greater risk for hospitalization among the hypersecretors. The association between frequent exacerbations and chronic bronchial hypersecretion was independent of other known risk factors for repeated exacerbations, such as FEV1, age, cardiovascular comorbidity or active smoking, which confirm chronic expectoration as a notable marker for exacerbation.
Bronchial hypersecretion has been associated with greater airway inflammation and greater risk for respiratory infection,59 which could explain the link with the appearance of repeated exacerbations. The same could be said of bronchiectasis, which is very frequently observed in patients with moderate-severe COPD and is also associated with increased expectoration, chronic bronchial infection and repeated exacerbations.60,61
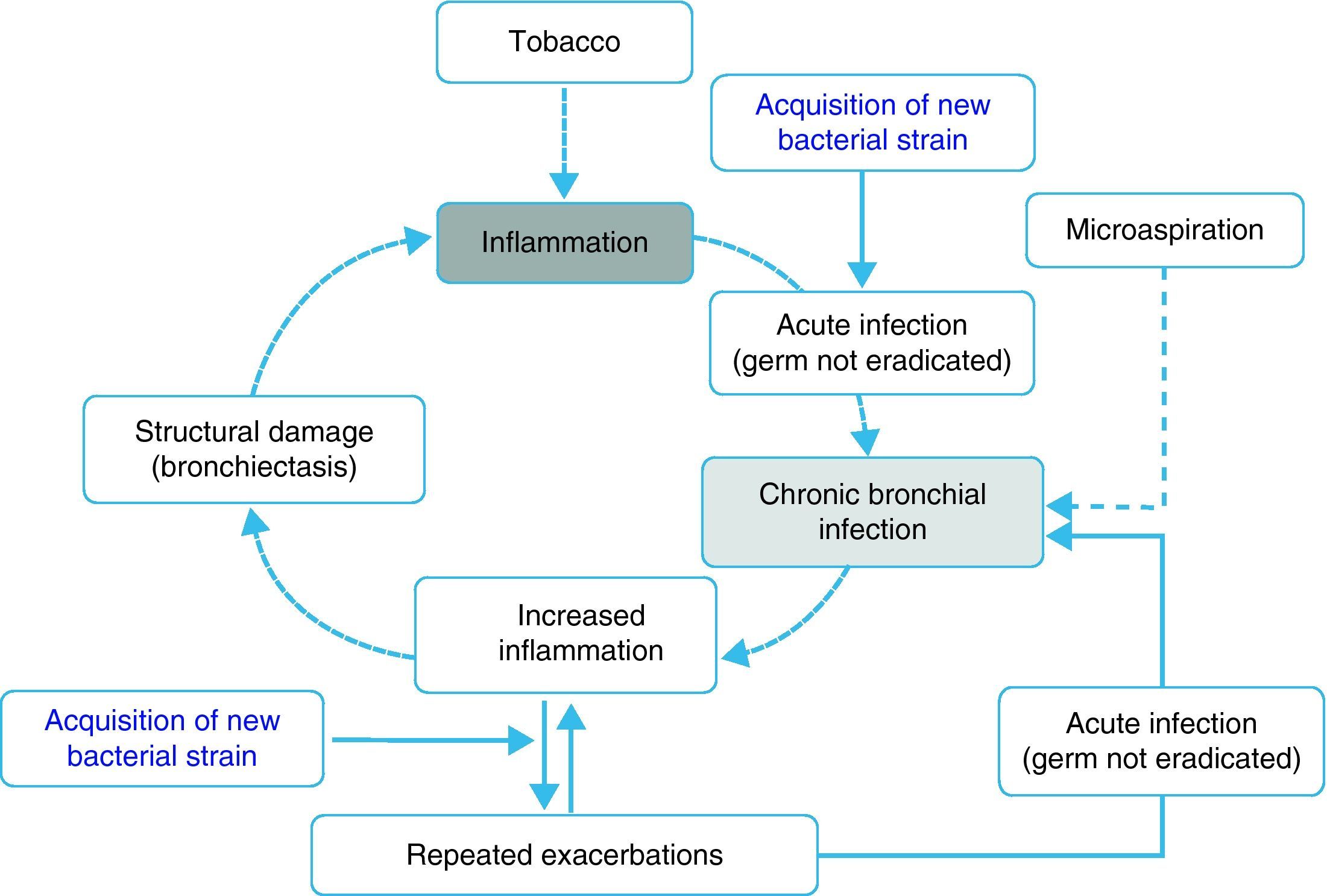
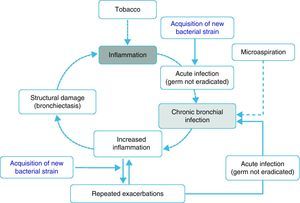
Inflammation, chronic bronchial infection, bronchiectasisSeveral studies have demonstrated that, during periods of stability, patients with frequent exacerbations present more airway inflammation and that this is regardless of tobacco habit as it persists even in ex-smokers with COPD.50,51,62 The cause of this greater inflammation has not been established; however, it has been postulated that in some patients it could be due to the presence of potentially pathogenic microorganisms (PPM) in the airway. The lower airway should be sterile, but, in close to 30% of clinically stable COPD patients, PPM are isolated.63 These germs can appear as a consequence of an acute infection that was not eradicated, or rather based on the presence of microaspiration. Traditionally, the presence of PPM in the lower airway is called “colonization”. However, these microorganisms are not innocuous; instead, they produce inflammation that, in addition, increases as the bacterial load and the frequency of exacerbations increase.51,62,64 It has been suggested that this bacterial load could increase over time and predispose the appearance of new exacerbations, surpassing a clinical threshold.65 Nevertheless, recent studies indicate that the exacerbation is also triggered after the acquisition of new bacterial strains66 and that the bacterial load does not always directly influence the development of an exacerbation. In this context, the most plausible hypothesis indicates that the greater inflammation, and perhaps the existence of some underlying structural alterations associated with it (bronchiectasis, for example), would create a favorable environment for the development of new exacerbations. The non-eradicated PPM (chronic bronchial infection) would in this way contribute to maintaining a vicious cycle, amplifying the underlying inflammation and inducing structural damage.67 In fact, bronchiectasis, frequently associated with the infection/bronchial-inflammation binomial, has also been recently linked to the existence of repeated and more severe exacerbations.60,61 Based on these arguments, some hypothetical models have been proposed that would support the inflammation-infection theory as an essential axis of the susceptibility to infectious exacerbation (Fig. 1). Antibiotic treatment, aimed at eradicating colonizing microorganisms as well as anti-inflammatory and immunomodulatory therapy, has been shown to reduce exacerbations, which backs the inflammatory-infectious hypothesis as an etiopathogenic factor that underlies in the exacerbator phenotype.
Viral infection can also play a relevant role in modulating the inflammatory response of the airway, altering the fragile balance between the presence of bacteria in the airway and the response of the host. In fact, patients with frequent colds (for example, viral infections by rhinovirus) also experience more bacterial exacerbations.68
Recently, it has been suggested that gastroesophageal reflux disease (GERD) may also predispose patients to frequent exacerbations.35,69 The intimate mechanism that links GERD and exacerbation is not clear, but some authors suggest the existence of alterations in the swallowing reflux and the possible existence of microaspiration,70 which once again reinforces the relationship between infection and the inflammation caused by repeated exacerbations.
Although in most exacerbations, especially if they are repeated, there is a potential underlying infectious mechanism, the truth is that the greater inflammation observed in exacerbator patients could have other origins. As mentioned in the previous section, there is a specific group of patients (mixed COPD-asthma phenotype) where certain asthmatic-type characteristics underlay. Recently, an observational, case–control study has shown evidence of a greater risk for suffering frequent exacerbations among patients who had an asthma diagnosis made by their physicians,52 which could suggest an alternative mechanism to the inflammatory-infectious one. However, the study was retrospective and observational; therefore the conclusions should be cautious. In addition, these patients with a reported diagnosis of asthma did not present greater reversibility in the bronchodilator test, nor did they have any objective measurements that confirmed the asthma diagnosis or, if not, the mixed asthma-COPD phenotype instead. Autoimmunity phenomena could also be connected with the persistence of greater airway inflammation. However, to date there is hardly any evidence to establish an association between said autoimmunity and the presence of repeated exacerbations.
Cardiovascular disease and repeated exacerbationsConfronting the inflammatory hypothesis (infectious or non-infectious), a significant association has been described among different cardiovascular pathologies and a greater frequency of exacerbations.36,40,46,54 In a prospective study with a case–control design in patients with severe COPD, the exacerbators presented a greater number of cardiovascular events than the subjects with COPD of similar severity, but without exacerbations.40 The direction of this association has not been clearly outlined. While some studies suggest that the exacerbations cause or trigger the cardiovascular manifestations through different mechanisms such as systemic inflammation, hypoxemia or endothelial dysfunction, it is not clear if it is the cardiovascular events themselves, such as some rhythm disorders (auricular fibrillation, flutter, etc.), episodes of myocardial ischemia or ventricular failure, those that could mimic an exacerbation with difficult differential diagnosis, due to, among other reasons, the non-specificity of the clinical symptoms. In fact, almost 30% of severe exacerbations present symptoms suggestive of heart failure,71 and we frequently observe higher levels of troponin, a marker of myocardial injury, during COPD exacerbations.72 Be they either a cause or consequence, the truth is that these cardiovascular episodes are especially relevant in severe exacerbations. In a series of patient deaths during hospitalization due to COPD exacerbation, heart failure was identified as the cause of death in more than one-third of the patients.73 Pulmonary embolism, which is also difficult to diagnose, explained more than 20% of the deaths.73
Individual Genetic SusceptibilityAlthough there is very little information available, the existence of a marked heterogeneity in the defense mechanisms of the host against the pathogen could indicate a certain genetic susceptibility. This fact has been supported by the finding of some polymorphisms in patients with frequent exacerbations. Differences in the genotype-dependent expression of the CCL1 protein, a chemotactic factor for the monocytes and macrophages, could produce alterations in the activation of the innate immune system versus respiratory infections.74 Likewise, polymorphisms have been described in the MBL2 (mannose binding lectin) connected with a greater frequency of hospitalizations.75 MBL is a protein of the innate immune system that inactivates a large number of microorganisms by means of the activation of the complement. Its deficiency, due to MBL2 polymorphisms, can potentially increase the susceptibility to infection.76
Greater Risk for Morbidity and MortalityTraditionally, COPD exacerbations have been considered clinical decompensations that are more or less transitory, whose repercussions were limited to the duration of the event itself. However, today we know that an important proportion of patients do not completely recover after an exacerbation, and this can cause later consequences, both pulmonary as well as systemic. In the cases in which repeated exacerbations are produced (exacerbator phenotype), the consequences can accumulate. Numerous studies have demonstrated the existence of a close relationship between the frequency of the exacerbations and the deterioration of the health-related quality of life (HRQL).38,40,77–79 The same is true with different extrapulmonary manifestations, such as depression, myopathy, myocardial infarction or GERD,39,53,54 much more frequent among the “exacerbator” patients. An accelerated deterioration in lung function has also been documented, which has been estimated at 8ml/year more among the patients with frequent exacerbations62 and even a persistent worsening of the BODE index.80 Finally, and perhaps as a consequence of all that has been mentioned, a poorer prognosis has been demonstrated because, as the frequency of exacerbations increases, so does the risk for death, regardless of the baseline severity of the disease.40,42 Thus, we believe that “exacerbator” patients form a special group of patients with a high risk for morbidity and mortality, whose therapeutic approach should be different and intensive. These patients are also an enormous burden for the health-care system as it is estimated that they are responsible for 60% of hospital services rendered.41
Differential TreatmentLong-acting bronchodilators, which are the first step in treating COPD, have been shown to reduce the frequency of exacerbations.81 When exacerbations persist despite bronchodilator treatment, the introduction of anti-inflammatories is indicated. In this context, various clinical practice guidelines7,82,83 recognize the usefulness of the use of IC in patients who present frequent exacerbation as their use, especially when associated with bronchodilators, produces a significant reduction in the number of exacerbations and an improvement in HRQL.31,32,84 Traditionally, this effect has been accepted for severe or very severe patients (FEV1<50%) with frequent exacerbations. However, some studies in patients with less functional severity also back the use of these drugs; therefore, it seems that the main determinant of the benefit is precisely the presence of repeated exacerbations.31 Roflumilast is a new oral anti-inflammatory drug that acts by selectively inhibiting phosphodiesterase IV which has been approved for preventing exacerbations in patients with severe COPD who present cough and chronic expectoration and also suffer frequent exacerbations85,86; therefore it is indicated for the exacerbator phenotype with chronic bronchitis. Macrolides, administered for a prolonged amount of time, could also have a specific indication for some of these patients as they have anti-inflammatory and immunomodulatory actions in addition to their possible antibacterial action.87 Some clinical assays suggest that the use of these drugs in stable patients with severe COPD significantly reduces the number of exacerbations, but with a possible increase in the risk of the appearance of bacterial resistances.88–91
Finally, and given the potential role of PPM, it has also been suggested that the use of antibiotics during periods of stability (antibiotic chemoprophylaxis or treatment of the chronic bronchial infection) could be useful for reducing exacerbations.92 In this direction, the PULSE (Pulsed moxifloxacin Usage and its Long-term impact on the reduction of Subsequent Exacerbation) study is a clinical trial that studied the efficacy of the administration of 5-day cycles of 400mg of moxifloxacin every 8 weeks in patients with stable COPD.93 The results indicate that this treatment reduced the risk for exacerbation by 20% in the intention-to-treat (ITT) analysis, 25% in the per-protocol (PP) analysis and 45% in patients who presented purulent or mucopurulent sputum, also by means of a PP analysis, without a significant increase in bacterial resistances. In another study done in patients with severe COPD colonized by Pseudomonas aeruginosa, the administration of nebulized tobramycin reduced the number of severe exacerbations by 42%, also reducing bronchial inflammation.94 More studies are required to help adequately profile candidate patients as well as the duration and the type of antibiotic treatment necessary. It is very likely that the patients who would most benefit from this option would be those who present frequent exacerbations and purulence in sputum during stable phases.
Diagnosis of the Exacerbator PhenotypeThe exacerbator phenotype is identified when the following criteria are met: existence of two or more exacerbations per year; the exacerbations should be separated at least 4 weeks after the end of the treatment of the previous exacerbation or 6 weeks from the start of the exacerbation in cases that have not received treatment.
In cases in which the exacerbator phenotype is finally established, it is necessary to properly characterize the patient, searching for the existence of chronic bronchial infection and/or the presence of bronchiectasis. The use of anti-inflammatory and/or antibiotics can be especially useful among these patients.
Emphysema-Hyperinflation PhenotypeIn recent years, many studies have demonstrated that variables such as dyspnea,95,96 exercise capacity97,98 and hyperinflation99 predict mortality independently from the lung function, and they are even better predictors than FEV1 itself. This justifies defining and establishing the emphysema-hyperinflation COPD phenotype as a group of patients with a higher risk for mortality which presents certain differences with regards to treatment guidelines.
Pulmonary emphysema is defined, in anatomopathologic terms, as the permanent destruction of the air spaces beyond the terminal bronchioles.3 We know that the loss of elastic retraction and the development of expiratory flow limitation make alveolar emptying difficult, originating air trapping and hyperinflation. This phenomenon has been associated with the limitations in the functional capacity of COPD patients100,101 and it is more closely related with dyspnea and tolerance to exertion than airflow obstruction. Moreover, it is known that the correlation between the extension and the severity of the macroscopic emphysema and the degree of obstruction (FEV1) is low.102,103 Nevertheless, the extension of the emphysema measured by HRCT does explain a large part of the variability of the carbon monoxide (CO) diffusing capacity.104 There are studies that have demonstrated an inverse correlation between body mass index (BMI) and degree of emphysema, evaluated by HRCT.8
Hyperinflation in patients with emphysema is usually divided into static and dynamic. Static hyperinflation is the most common, caused by the loss of retraction of the pulmonary parenchyma in patients with emphysema.105 Although we do not understand its development in the natural evolution of COPD, it appears more frequently and with greater intensity as the FEV1 diminishes. Dynamic hyperinflation can occur either independently or associated with static hyperinflation, and it appears in patients with any degree of severity.106 Dynamic hyperinflation is produced when inspiration begins before reaching complete expiration, which determines that with each breath a certain amount of air becomes trapped in the lungs. In patients with COPD, dynamic hyperinflation is produced when there is a limitation of expiratory airflow due to the airway obstruction, secondary to the increase in cholinergic tone, inflammation and mucus plugs. At the same time, it is favored by the increased collapsibility of the airways, which increases their resistance and prolongs the time necessary for completing the expiration. Hyperinflation entails an inspiratory load of the threshold type as in these patients the inspiration begins when there still has not been complete pulmonary emptying and the inspiratory muscles should first surpass the elastic retraction pressure of the lungs that still favors expiration (auto-PEEP or intrinsic PEEP). Hyperinflation is reversible in character; therefore, it is an attractive therapeutic target.
Definition of the Emphysema-Hyperinflation PhenotypeThe emphysema-hyperinflation phenotype defines COPD patients who present dyspnea and intolerance to exercise as the predominating symptoms, which are frequently accompanied by signs of hyperinflation. Patients with emphysema phenotype present a tendency towards a lower BMI.
This clinical form of COPD is characterized by the presence of functional data of hyperinflation, the existence of emphysema on the HRCT study and/or a diffusion test lower than the reference value, measured with the DLCO/VA ratio adjusted for hemoglobin. The presence of emphysema has not been associated with a greater risk for exacerbations, except if it coexists with chronic bronchitis107; in this case, the patient would be classified as exacerbator, and the treatment should prioritize reducing exacerbations.
Justification of the Emphysema-Hyperinflation PhenotypeGenetic SusceptibilityThe different phenotypic expression of the pulmonary disease in smokers is determined in part by genetic factors. Specifically, in family studies using HRCT, we have observed that there is family aggregation, regardless of the emphysema phenotype, which indicates the presence of genetic determinants that define this phenotype.108 More recent studies have identified single nucleotide polymorphisms (SNP) that are significantly associated with the extension of low-density areas in pulmonary HRCT,109 and even certain genetic loci have been significantly related with the presence and extension of the emphysema in large COPD patient populations.110 These studies justify the differentiation of the emphysema phenotype as a characteristic genetic-base process in smokers.
One special case is congenital emphysema due to alpha-1-antitrypsin deficiency, as it is produced by a genetic mutation in the gene that codifies this protein. Patients who are homozygote for the deficiency mutation have an increased risk for developing emphysema that is predominantly basal and early-onset.10 This type of emphysema has been used as a model for understanding the physiopathology of emphysema in smokers.
Greater Risk of Morbidity and MortalityThe clinical importance of identifying the emphysema-hyperinflation phenotype is based on the fact that the degree of dyspnea,95,96,111 intolerance to exercise95,111 and hyperinflation99 are predictors for mortality that are independent of the severity of the obstruction. In a prospective study with a 5-year follow-up, Casanova et al.99 observed an inverse relationship between the degree of hyperinflation and survival. They demonstrated that patients with COPD and an IC (inspiratory capacity)/TLC (total pulmonary capacity) ratio less of than 0.25 were 3.15 more likely to die than those with higher ratios. In this study, the multivariate analysis demonstrated that this ratio (IC/TLC) continued to be a risk factor, regardless of other parameters, such as FEV1, age, dyspnea, exercise capacity or comorbidity.
A positive relationship has also been demonstrated between the magnitude of the emphysema measured by HRCT, hyperinflation and the BODE index,112 although differences have not been observed in pulmonary attenuation among different quartiles of the BODE index, probably due to the participation of extrapulmonary factors in this prognostic score. Nevertheless, the presence of hyperinflation on HRCT in smokers with normal FEV1 is associated with a faster fall in FEV1113 and, finally, a significant association has been demonstrated between the magnitude of the emphysema evaluated by HRCT with a greater mortality in COPD, regardless of the severity measured by FEV1.114 In this manner, we see the growing evidence of the need for HRCT when evaluating COPD patients for the study of the emphysema as well as to evaluate the possible presence of bronchiectasis.
The impact of emphysema on mortality was also observed in the National Emphysema Treatment Trial (NETT),115 in which the 3 factors—emphysema, hyperinflation and the BODE index—were independent predictors of mortality. However, we should be reminded that this is a cohort of patients with very severe COPD and, therefore, these results are not extrapolatable to all COPD patients. These data are corroborated by studies such as that by Nishimura et al.96 and the one by Martínez et al.,95 where the patients with higher residual volume had a greater mortality, with a similar tendency in cases where the IC/TLC ratio was lower.
Indirectly, dynamic hyperinflation can contribute to a poorer prognosis of COPD by significantly reducing the exercise capacity of patients who are affected by it. Thus, it has been demonstrated that physical activity in patients with moderate-to-severe COPD correlates inversely with degree of dynamic hyperinflation,116 and the patients with less physical activity are those who present a higher rate of hospitalizations and greater mortality.117
Cardiovascular Disease and EmphysemaPulmonary hyperinflation can have an effect on the size of the heart and its function. Several studies associate hyperinflation and the presence of diastolic dysfunction. The study by Vassaux et al.118 demonstrates that the cardiac oxygen pulse, as an overall measurement of cardiac function during an exertion test, is lower in patients with COPD and hyperinflation, measured with an IC/TLC ratio ≤0.25. In the study by Jörgensen et al.,119 it was also observed that the size of the right and left ventricles is smaller in patients with severe emphysema and it is accompanied by a decrease in the filling of the left ventricle, a reflection of the reduction of the preload secondary to pulmonary hyperinflation. Recently, the study by Watz et al.120 analyzed the relationship between lung function alterations and echocardiographic measurements of cardiac function and size in COPD patients with different degrees of severity. The results show that hyperinflation (IC/TLC) is significantly associated with the telediastolic diameter of the left ventricle, much better than with the degree of airflow obstruction. Thus, patients with an IC/TLC ratio ≤0.25 had diastolic dysfunction of the left ventricle with overall affectation of the function of the right ventricle, and this was associated with less tolerance for exercise. Also, a population study demonstrated that the degree of emphysema, evaluated by HRCT, was linearly related with the affectation of left ventricle filling and a decrease in the cardiac output.121 These data suggest that the treatment directed at reducing hyperinflation can have a direct impact on the cardiac function and exercise capacity, and at the very least they could partially explain the tendency towards a reduction in mortality with long-acting bronchodilators observed in the large clinical assays with these drugs in COPD.31,122,123
Differential Treatment of the Emphysema-Hyperinflation PhenotypeThe presence of hyperinflation, given its reversible character, can be a therapeutic target for bronchodilators. We know that hyperinflation measured by IC has been shown to be a reliable parameter in the evaluation of the response to some treatments and more sensitive than FEV1 in capturing the possible beneficial effect of some therapeutic options of COPD. Several studies have demonstrated improvements in forced vital capacity (FVC) after the administration of a bronchodilator in patients with moderate or severe COPD and hyperinflation, with improvements in IC and reduction in air trapping, but with no significant improvements in FEV1.124,125 This improvement in the volume without changes in flow is more frequent as the bronchial obstruction becomes more severe. The NETT study115 also did not demonstrate the superiority of the surgical intervention versus conservative treatment, but it did show that, in patients who presented emphysema in the upper lobes and low exercise capacity, a significant reduction in mortality was achieved after lung reduction surgery. In addition, the improvement in lung function after surgery was accompanied by a significant reduction in the number of exacerbations and prolonged the exacerbation-free time.126
According to the current guidelines, long-acting bronchodilators are the foundation of the pharmacological treatment of COPD. They improve symptoms and exercise capacity and, consequently, improve the state of health as perceived by the patient, with statistically significant and clinically relevant changes.127 Nevertheless, occasionally the benefits reached at the clinical level do not translate into an improvement of the degree of obstruction (changes in FEV1) but of hyperinflation instead by reducing dynamic hyperinflation with improvements in the inspiratory capacity, the degree of dyspnea and exercise tolerance.127–129
The current guidelines recommend the association of bronchodilators in order to try to achieve an additional effect, without increasing the adverse effects in the patients with poorly controlled symptoms in spite of treatment with a bronchodilator. In this direction, the use of double bronchodilator therapy (formoterol and tiotropium) versus bronchodilator monotherapy130 or versus the fluticasone-salmeterol combination131 offers an added functional benefit with reduction of the need for rescue medication, improvement in the symptoms and quality-of-life questionnaires. These results can be applied to other LABA/IC combinations.
Anti-inflammatory treatment with inhaled corticosteroids, whose main objective is the prevention of exacerbations, has not been shown to be as effective in the emphysema-hyperinflation phenotype.21 Nor has the oral anti-inflammatory roflumilast offered good results for the reduction of exacerbations in patients with emphysema, except in those who associated symptoms of chronic bronchitis.132
In short, patients with an emphysema-hyperinflation phenotype could benefit more from a double bronchodilator therapy and, of course, from respiratory rehabilitation due to its beneficial effects on dyspnea and exercise tolerance.133
Diagnosis of the Emphysema-Hyperinflation PhenotypeThe lung function parameter that best evaluates the presence of emphysema is the carbon monoxide transference capacity (DLCO), which correlates well with the severity of pulmonary emphysema.134 Nevertheless, one of its limitations is that it analyzes the entire lung as a whole, unlike HRCT, which is able to detect localized destructive changes, and currently this imaging technique is often used for detecting pulmonary emphysema. In addition, recent studies show that quantifying the magnitude of pulmonary emphysema using densitometry parameters could be a sensitive and specific exploration in the evaluation and the follow-up of pulmonary emphysema.135 Thus, there are studies that have demonstrated that the analysis of the density of the pulmonary parenchyma in HRCT correlates with the pathological alterations observed in tissue samples136,137 and with lung function deficiency (airflow obstruction and diffusion capacity),138,139 which would allow for a radiological estimation of the COPD severity.
Hyperinflation is evaluated by means of the determination of static lung volumes. Nevertheless, IC obtained with slow spirometry provides an indirect estimation of the magnitude of the hyperinflation in a simpler, reproducible manner.140 IC correlates well with dyspnea and with exercise capacity in COPD patients.141 It has been observed that the reduction in IC correlates with an increase in dyspnea and a decrease in exercise capacity.142 This fact is justified because the most important physiopathological factor that determines exertion dyspnea in COPD patients is the development of air trapping and dynamic hyperinflation triggered by exercise, including the physical exertion associated with carrying out the activities of daily life.116
ConclusionsCOPD is a heterogeneous disease, but for many years patient peculiarities have not been taken into account when recommending treatment. Mostly, this has been due to the fact that there were few therapeutic options and that there was no evidence that their effectiveness was significantly different in different types of patients. The development of different options for pharmacological and non-pharmacological treatments has led to the demonstration that the clinical response can be different according to the characteristics of the disease. The concept of phenotype applied to COPD has resulted in the definition of different types of patients with prognostic and therapeutic significance. In this way, we may take on a more personalized treatment according to not only the severity of the airflow obstruction, but also conditioned by the clinical phenotype.28,29,143,144 In this review, we give reasons for considering three fundamental phenotypes: exacerbator, mixed and emphysema. It is evident that not all the patients will meet the criteria to be able to classify them unequivocally into one of the subgroups, and it will always be the physician's clinical judgment which will classify the patient into the most relevant phenotype for its prognosis. In this direction, the simple question of, “How many exacerbations did the patient have the previous year?” will classify the patient as an exacerbator if the answer is “two or more”, whatever the clinical or functional characteristics of the patient, as the treatment should prioritize the prevention of exacerbations. If the response is “one or none”, we should confirm whether the patient is either an emphysema or mixed phenotype. The following step will be to recognize these clinical phenotypes in the new COPD treatment guidelines.145
Conflict of InterestsMarc Miravitlles has received professional fees for scientific consulting and/or for giving conferences from Almirall, AstraZeneca, Bayer Schering, Boehringer Ingelheim, Grupo Ferrer, GlaxoSmithKline, Laboratorios Esteve, Pfizer, Novartis, Merck Sharp & Dhome and Nycomed. Myriam Calle has received professional fees for giving conferences from Almirall, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Novartis, Merck Sharp & Dhome and Nycomed. Juan José Soler Cataluña has received professional fees for scientific consulting and/or for giving conferences from Almirall, AstraZeneca, Boehringer Ingelheim, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Pfizer, Novartis, Merck Sharp & Dhome and Nycomed.
Please cite this article as: Miravitlles M, et al. Fenotipos clínicos de la EPOC. Identificación, definición e implicaciones para las guías de tratamiento. Arch Bronconeumol. 2012;48:86-98.