Cigarette smoke is the main cause of inflammation in COPD. The mechanisms that differentiate smokers who develop COPD are diverse. In this study, we analyzed the presence of cytokines in the respiratory secretions of smokers with or without COPD and the secretory properties of the differentiated bronchial epithelium obtained from the individuals themselves after exposure to tobacco smoke.
Material and methodsTwenty-seven smokers were studied, 12 of whom had COPD that had not been previously treated with steroids. In 11, samples were obtained by means of induced sputum, and the remaining samples were collected from bronchial aspiration after bronchoscopy. Concentrations of IL8, IL13, and TNFα in the supernatant were determined. The results obtained were compared between individuals with and without COPD, and we studied their relationship with the severity of COPD as expressed by the degree of obstruction, dyspnea, presence of hypersecretion and intensity of smoking. Bronchial epithelial cell cultures were obtained by air–liquid interface in 4 smokers. The samples were exposed to increasing concentrations of cigarette smoke (5%–20%) and the epithelial mRNA expressions of Muc5AC, IL8, and TNFα were determined.
ResultsCOPD patients had significantly higher values of IL8 than healthy smokers (41 [22]pM vs 21 [12]pM). The values of IL8 correlated significantly with the severity of the obstruction (r=0.6; P<.05), dyspnea (r=0.45; P<.05) and the presence of hypersecretion. There was no relationship between cytokines and the intensity or duration of the tobacco habit. Cigarette smoke produced a dose-dependent increase in the expression of RNAm for Muc5AC, IL8, and TNFα.
ConclusionsThere are differences in cytokine production (fundamentally IL8) between smokers and smokers with COPD which could be explained by the direct action of cigarette smoke on epithelial cells.
El humo de tabaco es la principal causa de la inflamación en la EPOC. Los mecanismos que diferencian a los fumadores que desarrollan EPOC son diversos. En este estudio analizamos la diferente presencia de citocinas en secreciones respiratorias de pacientes fumadores con o sin EPOC y las propiedades secretoras del epitelio bronquial diferenciado, obtenido de los propios individuos tras su exposición al humo de tabaco.
Material y métodosSe estudió a 27 pacientes fumadores, 12 de ellos con EPOC no tratados previamente con esteroides. En 11 se obtuvo la muestra mediante esputo inducido y el resto procedía del aspirado bronquial tras fibrobroncoscopia. Se determinaron las concentraciones de IL8, IL13 y TNFα en el sobrenadante. Se compararon los resultados obtenidos entre individuos con o sin EPOC y se investigó su relación con la gravedad de la EPOC expresada según el grado de obstrucción, disnea, presencia de hipersecreción e intensidad del tabaquismo. Se obtuvieron cultivos de células diferenciadas epiteliales bronquiales, mediante interfase aire-líquido en 4 individuos fumadores. Las muestras fueron expuestas a concentraciones crecientes de humo de tabaco (5–20%) y se determinó la expresión epitelial de ARNm de Muc5AC, IL8 y TNFα.
ResultadosLos pacientes con EPOC tenían valores significativamente más altos de IL8 que los fumadores sanos (41 [22] vs. 21 [12] pM). Los valores de IL8 se correlacionaron de forma significativa con la gravedad de la obstrucción (r=0,6; p<0,05), disnea (r=0,45; p<0,05) y la presencia de hipersecreción. No había relación entre las citocinas y la intensidad o duración del hábito tabáquico. El humo de tabaco produjo un incremento dependiente de la dosis en la expresión de ARNm para Muc5AC, IL8 y TNFα.
ConclusionesExisten diferencias en la producción de citocinas, fundamentalmente IL8, entre individuos fumadores sanos o con EPOC que podrían ser justificadas por la acción directa del humo de tabaco sobre las células epiteliales.
Tobacco smoke is the main cause of chronic inflammation in the airways of individuals who smoke, which persists even after smoking cessation. The inflammation is mainly characterized by structural changes in the bronchial epithelium and an increase in cell infiltration by CD8 lymphocytes, neutrophils, and macrophages.1,2 The intensity of these alterations is greater in individuals that develop chronic obstructive pulmonary disease (COPD) than in healthy smokers3,4 and differs according to the predominant phenotype. The prevalence of COPD between smokers is, however, very variable and the mechanisms that condition its appearance have still not been clarified.
Recent studies that analyze the combination of genetic, environmental, and immunological factors indicate that the differences in the incidence and phenotypic expression of COPD can be caused by structural and functionally different responses depending on the characteristics of the local inflammatory medium and variations in individual susceptibility to tobacco.5,6 In this regard, analyses of inflammatory biomarkers obtained from airway secretions, both in vivo in smoker subjects with or without COPD7 as well as in vitro from cell cultures stimulated with tobacco smoke, have demonstrated increased expression, production, and release of proinflammatory cytokines, including: IL8, IL13, and TNFα,8–10 as well as other mediators of cell destruction, such as elastase, metalloproteinase, and free radicals derived from oxidative stress.11,12
The interaction between these substances and the cell structures is complex and variable in each individual and can explain the differing susceptibility and diversity of alterations observed in patients with COPD. Among the most frequent phenotypes are patients with bronchial hypersecretion. The mechanisms that lead to this exaggerated production are fundamentally the overexpression of mucin genes, hypertrophy or hyperplasia of the secretory glands and cells or an increase in the release of mucus. The mucin detected in the airways are a mix of oligomeric glycoproteins, fundamentally Muc5AC and Muc5B, which are synthesized and stored in the apical surface of the bronchial epithelium and released13,14 as a consequence of the action of proinflammatory cytokines, bacterial exoproducts, or the neutrophilic elastase itself. The mechanism of action common to all these stimuli is produced through EGFR (epidermal growth factor receptor).15–17 In other COPD patients, the predominant phenotype is due to the changes in cell destruction and repair18 that condition the appearance of emphysema. In this group, the intervention of cytokines has been observed, such as IL8, IL13, and TNFα, in cell apoptosis19 and an increase in fibrogenic growth factors such as collagen or fibronectin.20,21
The way in which each one of these mechanisms predominates in response to tobacco smoke is unknown. In this research field, the studies are limited and have mostly been done in cultures of undifferentiated epithelial cell lines obtained from healthy individuals and not from smoker patients with or without COPD. The hypothesis proposed is that in smoker patients with COPD there should be a higher expression of these cytokines and mucins as a consequence of the direct action of smoking on the epithelial cells.
In this present study, we have therefore set forth two fundamental objectives. The first of these was to research the differing production of IL8, IL13, and TNFα in respiratory secretion samples from smokers with or without COPD, analyzing the existing correlation with the clinical and functional parameters that characterize COPD. The second objective was to study the secretory properties of differentiated bronchial epithelial cultures obtained from the individuals themselves after their stimulation by means of exposure to tobacco smoke.
Patients and MethodsStudy SubjectsWe studied a group of adult individuals, aged from 18 to 70, whose main requisite was a history of active smoking of at least 10 pack-years. The subjects were mostly recruited from the hospital tobacco treatment department. In the case of the group that agreed to bronchial biopsy, we included patients that required bronchoscopy due to having presented hemoptoic sputum and after ruling out infectious or neoplastic causes.
To be included in the study, the patients needed to have been stable, with no episodes of exacerbation in the previous 2 months, and to never have been treated with inhaled anticholinergics and/or steroids, or with mucolytics or oral antioxidants.
Excluded from the study were all those patients that presented any chronic lung disease other than COPD, severe systemic disease or localized bronchopulmonary neoplasm.
The study was approved by the hospital's Ethics and Research Committee.
Study DesignOnce the inclusion criteria was met and the absence of excluding factors had been demonstrated, the patients were informed of the volunteer nature of the study and its aims, and informed consent was obtained.
From all the patients, we collected data for anthropometric, clinical, radiological, and respiratory function test characteristics. Among the clinical variables analyzed were predominant symptoms, degree of dyspnea measured according to the Medical Research Council (MRC) scale and the presence or of signs of right heart failure. Testing included chest radiography or computed tomography and lung function tests, including forced spirometry with a bronchodilator test, lung volume, and diffusion capacity determinations. Likewise and before spirometry, the expired fraction of nitric oxide (NO) was determined in exhaled air (FeNO) by means of a chemiluminescence analyzer (Logan Research, model 2000). The results were expressed in parts per billion. The determination of FeNO has been proposed as an indirect measurement of oxidative stress in patients with COPD or bronchiectasis.
Quantification of Cytokines in Respiratory SecretionsThe airway secretion samples were obtained by means of either induced sputum or bronchial aspiration during bronchoscopy. In this latter group, the exploration was used to obtain a mucus biopsy in the main bronchi from a macroscopically normal area. In the first group, the sputum sample was obtained by means of ultrasound nebulization with hypertonic saline solution in accordance with the established methodology for inducing sputum. Processing of the sputum samples and bronchial aspiration samples was done immediately in the following manner: using tweezers, the mucus accumulations were separated from the saliva portion and stored in two separate polypropylene tubes; one tube was used for cell determination and the other for determining concentrations of compositions in solid phase.
The portion intended for the cell count was homogenized with 10% dithiothreitol (DTT) solution with a concentration four times the volume of the initial sample. Later, phosphate buffer was added at 1:1 volume dilution and was filtered through a 42μm pore mesh. From this filtered sample, 0.4ml was obtained in order to determine total cell count and cell viability. The cell pellet obtained was resuspended in phosphate buffer at a volume sufficient to adjust the sample to 106 cells and to be able to take the differential cell count by means of Giemsa staining. In the sample, it was necessary for at least 400 inflammatory cells to be counted and for there to be less than 50% epithelial cells. The supernatant was frozen at −70°C for the later determination of its components.
The sample for the study of the soluble markers (TNFα, IL13, and IL8) was not homogenized with DTT as this substance destroys the disulphide bridges present in the interleukins. The sample was centrifuged directly at 60000×g for 90min and the supernatant portion was stored at −70°C for later determination.
Both sputum samples as well as a bronchial aspiration samples were sent for staining and quantified culture, which were to be rejected in cases of bronchial infection.
The concentrations of TNFα (eBIOSCIENCES, 88-7346-22), IL8 (DIACLONE 850050096), and IL13 (Cayman Chem, United States) in the supernatant were determined by means of an enzyme immunoanalysis kit, following the instructions of the supplier. In short, the samples were deposited in microplates and incubated at 40°C for 24h with monoclonal antibodies; after washing, the temperature was set at 37°C for 1h. The photometric reading was done at a wavelength of 450nm. The limits for detection and the sensitivity threshold for each measurement were established following the data of the supplier.
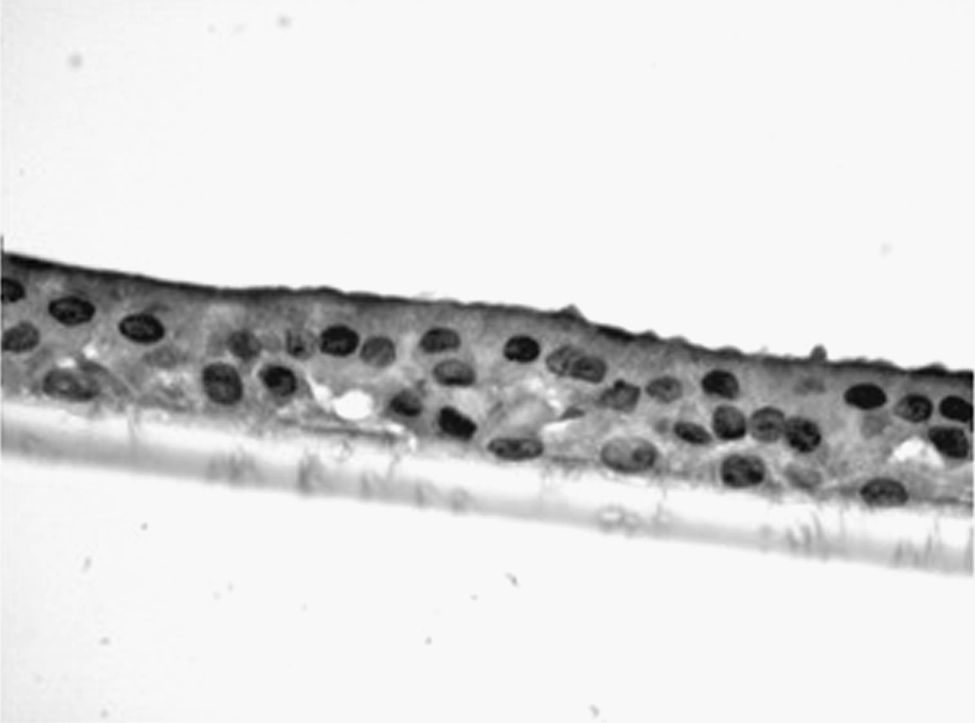
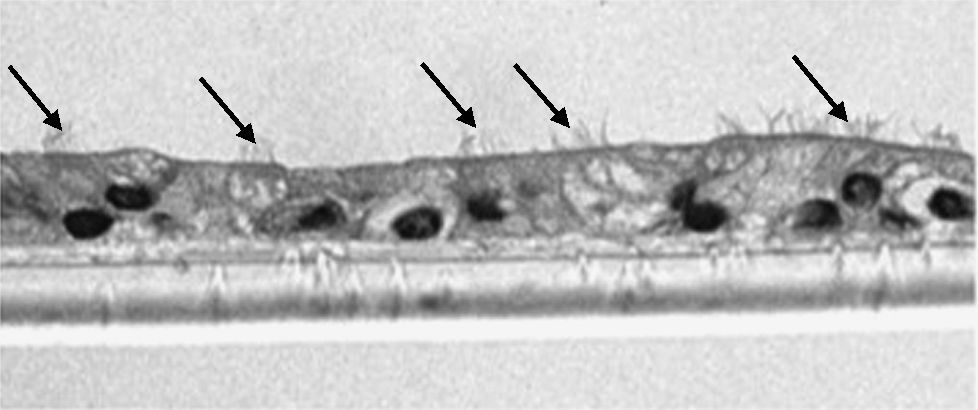
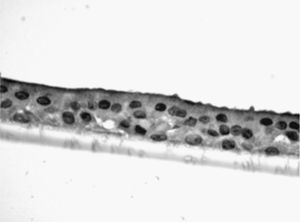
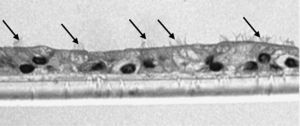
Study of the Response to Tobacco Smoke in Cultures of Differentiated Epithelial CellsThe bronchial biopsy samples were deposited in a cell culture medium, where they were maintained until plaque of converging primary epithelial cells were formed. Once these cell plaques were obtained, they were deposited in culture inserts (8.25×104 cells/insert) (Transwell, Corning Costar, Buckinghamshire, United Kingdom) with a differentiation medium (50% DMEM), epithelial cell basal medium (Clonetics, United Kingdom) for 7 days until they converged. Afterwards, the cells were trypsinized and newly cultured until they converged. This maneuver was repeated on 2 or 3 occasions. After this period, the cells were cultured for another 21 days in air–liquid interface (ALI technique), with the apical surface in contact with the air and the basal surface submerged in growth medium, until achieving differentiation in pseudostratified epithelium with presence of ciliated and caliciform cells. This characteristic was demonstrated by analyzing the samples in an inverted microscope. In random samples and after immersion in paraffin, staining was begun by means of immunohistochemistry with cytokeratin in order to demonstrate the epithelial nature, and with PAS-Schiff for the study of the mucus secretions.
Once differentiated, the cell samples were subjected, separately in each insert, to incremental exposure to cigarette smoke extract at varying concentrations (5%, 10%, 15%, and 20%) for 6 and 24h.
The tobacco smoke extract was obtained by combusting filterless cigarettes in a modified pump that allowed the smoke to bubble through 25ml of culture medium that was a mix of RPMI-1640 (Gibco Life Technologies) and LHC-D (Biofluids) at a ratio of 1:1. The pH of the resulting suspension was adjusted to 7.4 with NaOH and filtered through 0.22μm, then applied to the cell cultures within 30min of preparation. To see the effect of the volatilization, the tobacco smoke can be lyophilized and then recomposed in the same volume of distilled water.
The study of tobacco smoke in the production of mucins and cytokines in the cell cultures was analyzed by the determination of RNAm for Muc5AC, IL8 and TNFα, measuring their concentration at baseline situation and after stimulation with tobacco smoke for 6 and 24h. At the end of the exposure time, we proceeded with the cell destruction and extraction of RNA under each experimental condition.
As a quantitative technique for evaluating the quantity of RNAm, we chose real time-PCR (Applied Biosystem), expressing the results as ratios compared with the GAPDH control gene. Said technique is based on converting the RNA from the epithelial cells in this case into DNAc (complementary DNA) in order to carry out a monitored, real time-PCR (thanks to the increase in fluorescence that is generated by the dissociation of the probe due to the exonuclease function of the DNA polymerase). Once said kinetic is obtained, an increase in fluorescence is chosen for which the slope of the curve is linear in all the reactions (Ct). The reaction was done in an ABIPrism 7700 thermocycler (Perkin-Elmer). The analysis of the reactions was calculated with SDS software (Sequence Detection System) by Perkin-Elmer.
Statistical AnalysisThe data are expressed as means (SD) in the case of quantitative variables or in percentage in the case of qualitative variables. The comparative study of cytokine values in smoker patients with or without COPD was done by means of the Wilcoxon test and the correlation with the functional or clinical parameters by means of Spearman's correlation analysis. In the case of the experimental study of the differences in the proportion of mucins or cytokines before and after the stimulation with different concentrations of tobacco smoke, the non-parametric variance analysis for repeat samples was used. Given the nature and experimental difficulty of the study and the absence of previous reference values to estimate the necessary sample of biopsies, the calculation of the sample size was done with the intention of finding differences of at least 10% in the values of cytokines in sputum, similar to the Keatings study7 in smoker patients with or without COPD, assuming a power of 90%, an alpha value of 5% and a loss of 25%. The analyses were done using the SPSS 11.1 statistical package. The level of significance was P<.05.
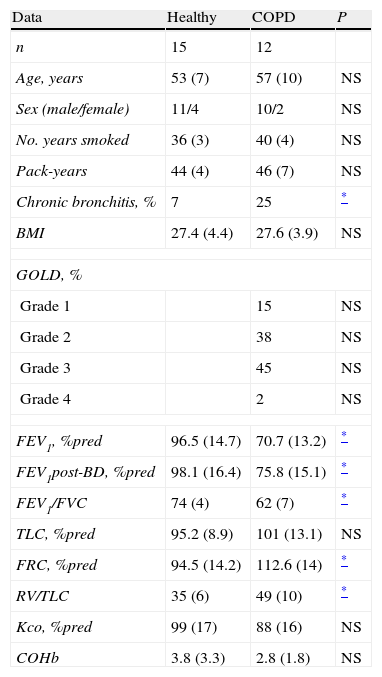
ResultsIncluded for study were 27 smoker individuals, 15 of them healthy and 12 diagnosed with COPD. The epidemiologic, clinical, and functional characteristics are shown in Table 1. There were no significant differences between the two groups regarding age, sex, intensity, and duration of tobacco habit or body mass index. The distribution of the severity of COPD according to the GOLD criteria, logically, was only applicable to the COPD group, and shows a predominance of obstruction in grades 2–3. The percentage of patients who referred cough and/or expectoration with criteria for chronic bronchitis was also significantly higher in the COPD group. The functional values, due to the inclusion criterion, were significantly lower in the second group.
Differences in the Anthropometric, Clinical and Functional Characteristics of the Smoker Patients With or Without COPD.
| Data | Healthy | COPD | P |
| n | 15 | 12 | |
| Age, years | 53 (7) | 57 (10) | NS |
| Sex (male/female) | 11/4 | 10/2 | NS |
| No. years smoked | 36 (3) | 40 (4) | NS |
| Pack-years | 44 (4) | 46 (7) | NS |
| Chronic bronchitis, % | 7 | 25 | * |
| BMI | 27.4 (4.4) | 27.6 (3.9) | NS |
| GOLD, % | |||
| Grade 1 | 15 | NS | |
| Grade 2 | 38 | NS | |
| Grade 3 | 45 | NS | |
| Grade 4 | 2 | NS | |
| FEV1, %pred | 96.5 (14.7) | 70.7 (13.2) | * |
| FEV1post-BD, %pred | 98.1 (16.4) | 75.8 (15.1) | * |
| FEV1/FVC | 74 (4) | 62 (7) | * |
| TLC, %pred | 95.2 (8.9) | 101 (13.1) | NS |
| FRC, %pred | 94.5 (14.2) | 112.6 (14) | * |
| RV/TLC | 35 (6) | 49 (10) | * |
| Kco, %pred | 99 (17) | 88 (16) | NS |
| COHb | 3.8 (3.3) | 2.8 (1.8) | NS |
COHb: carboxyhemoglobin; GOLD: global initiative for chronic obstructive lung disease; IC/TLC: ratio between inspiratory capacity and total lung capacity; BMI: body mass index; NS: not significant; RV: residual volume; TLC: total lung capacity.
Data expressed as mean (SD) in the quantitative variables and number of patients (frequency) in the qualitative variables.
The respiratory secretion samples were obtained in 11 patients by means of induced sputum and in the remaining 15 in the bronchial aspiration obtain after bronchoscopy. There were no significant differences in the distribution of patients included in each group depending on the place the sample was obtained.
The differential count of the cell component from the induced sputum or bronchial aspiration samples, in absolute values as well as in percentage of each cell type, showed no significant differences between both groups. In none of the samples was bacterial contamination observed that could interfere with the results.
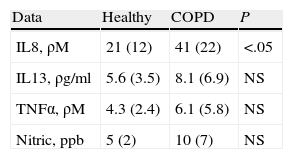
Regarding the analysis of the differing production of IL8, IL13, and TENα in the samples obtained from both patient groups, the data obtained from the supernatant of the sputum and bronchial aspiration showed that the smoker patients with COPD had significantly higher values of IL8 than healthy smokers (Table 2), while there were no differences in the values of TNFα or IL13. Given that some studies have shown differences in the inflammatory marker values mainly in asthma patients after the repeated induction of sputum, we compared the results obtained in the bronchial aspiration and in induced sputum without observing significant differences. Nor were there differences in the values of nitric oxide in exhaled air.
The values of IL8 correlated significantly in COPD patients with FEV1 before the bronchodilator (r=0.55; P<.05), FEV1/FVC (r=0.6; P<.05), dyspnea (r=0.45; P<.05) and the presence of chronic bronchitis (r=0.56; P<.05). There was not, however, a relationship between IL8, IL13, or TNFα with the intensity or duration of tobacco habit, either in healthy smokers or in individuals with COPD.
Out of the 15 patients who underwent bronchoscopy, only 7 agreed to a biopsy being taken of their bronchial mucus (4 healthy smokers and 3 with COPD).
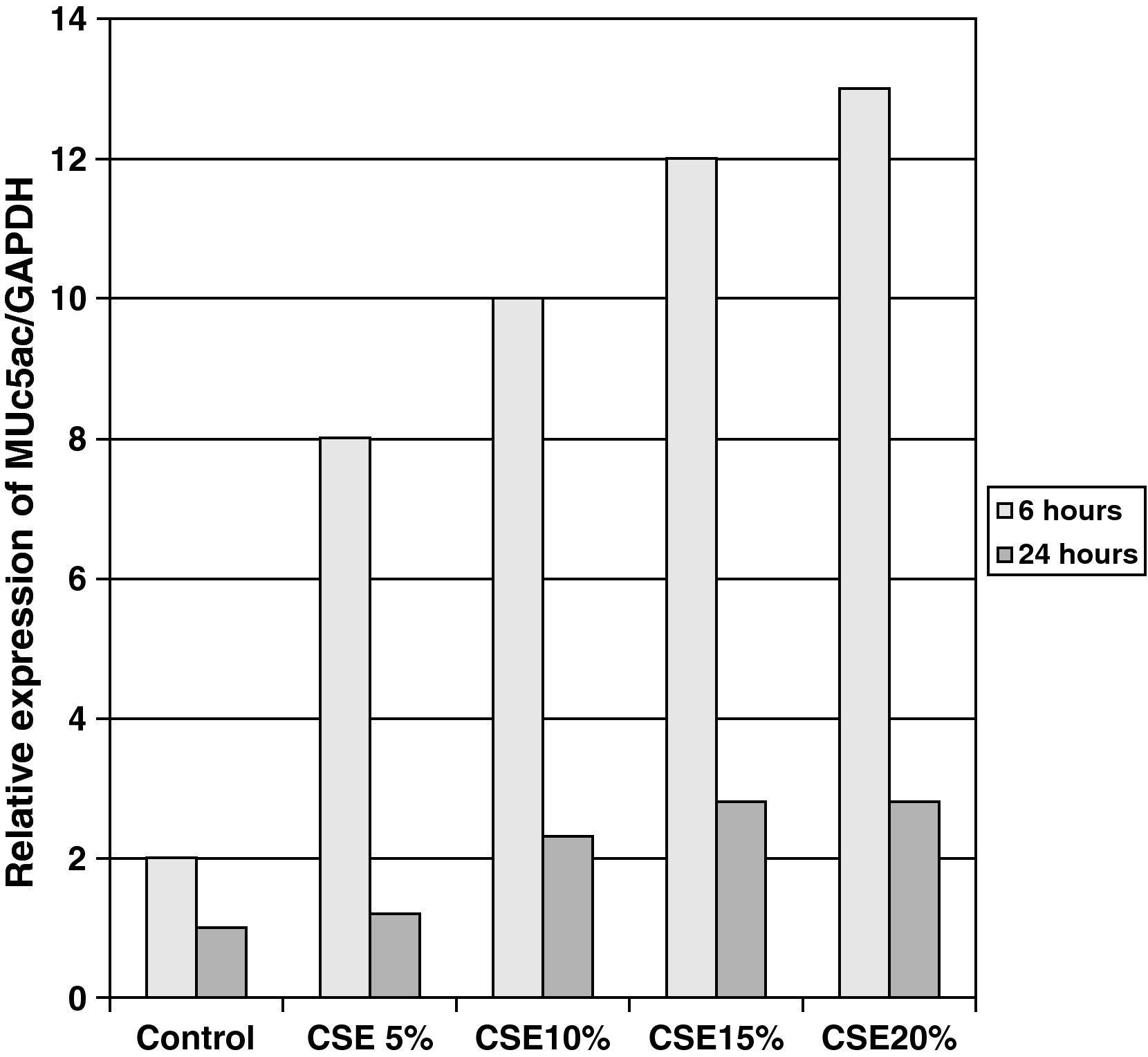
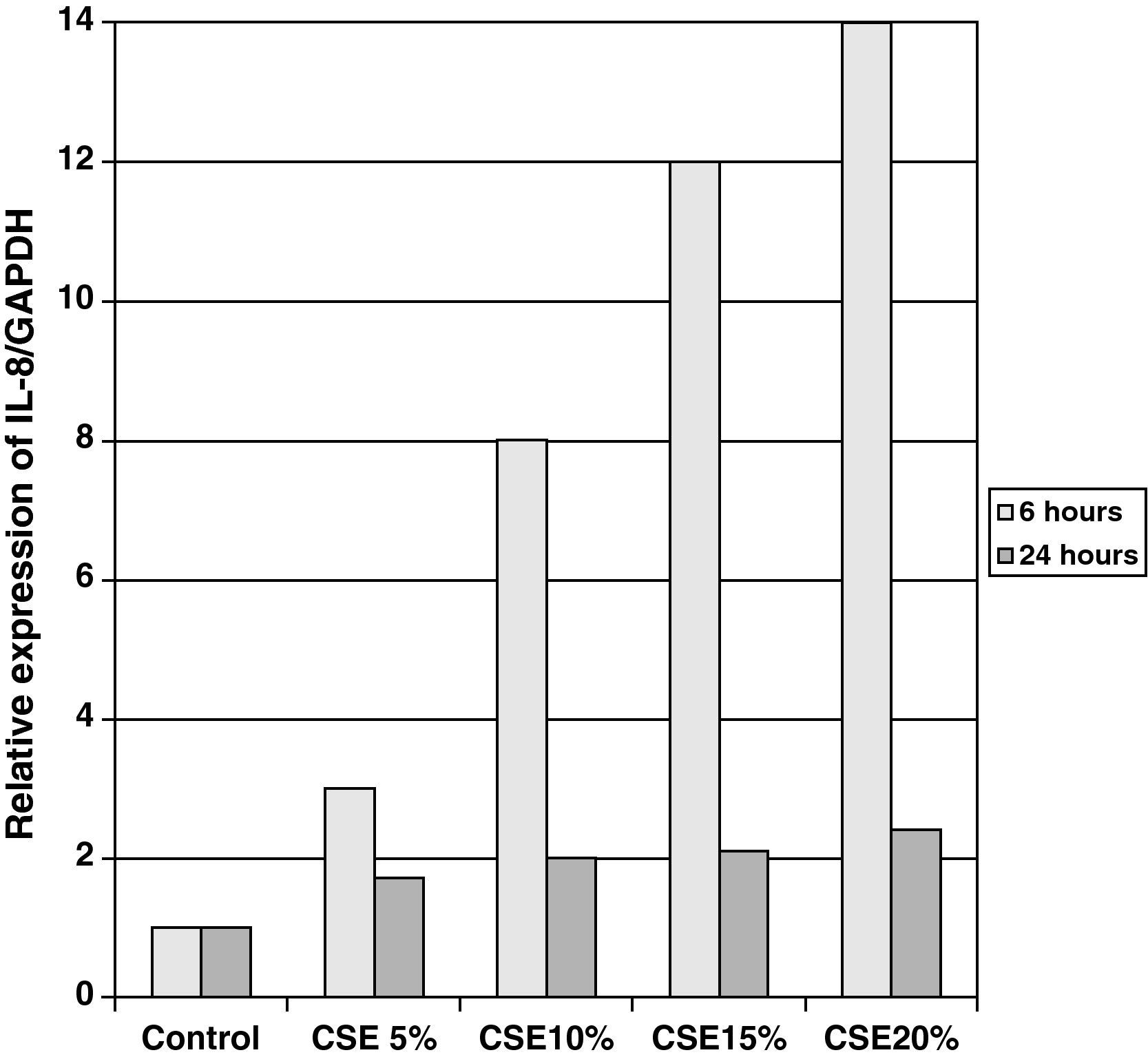
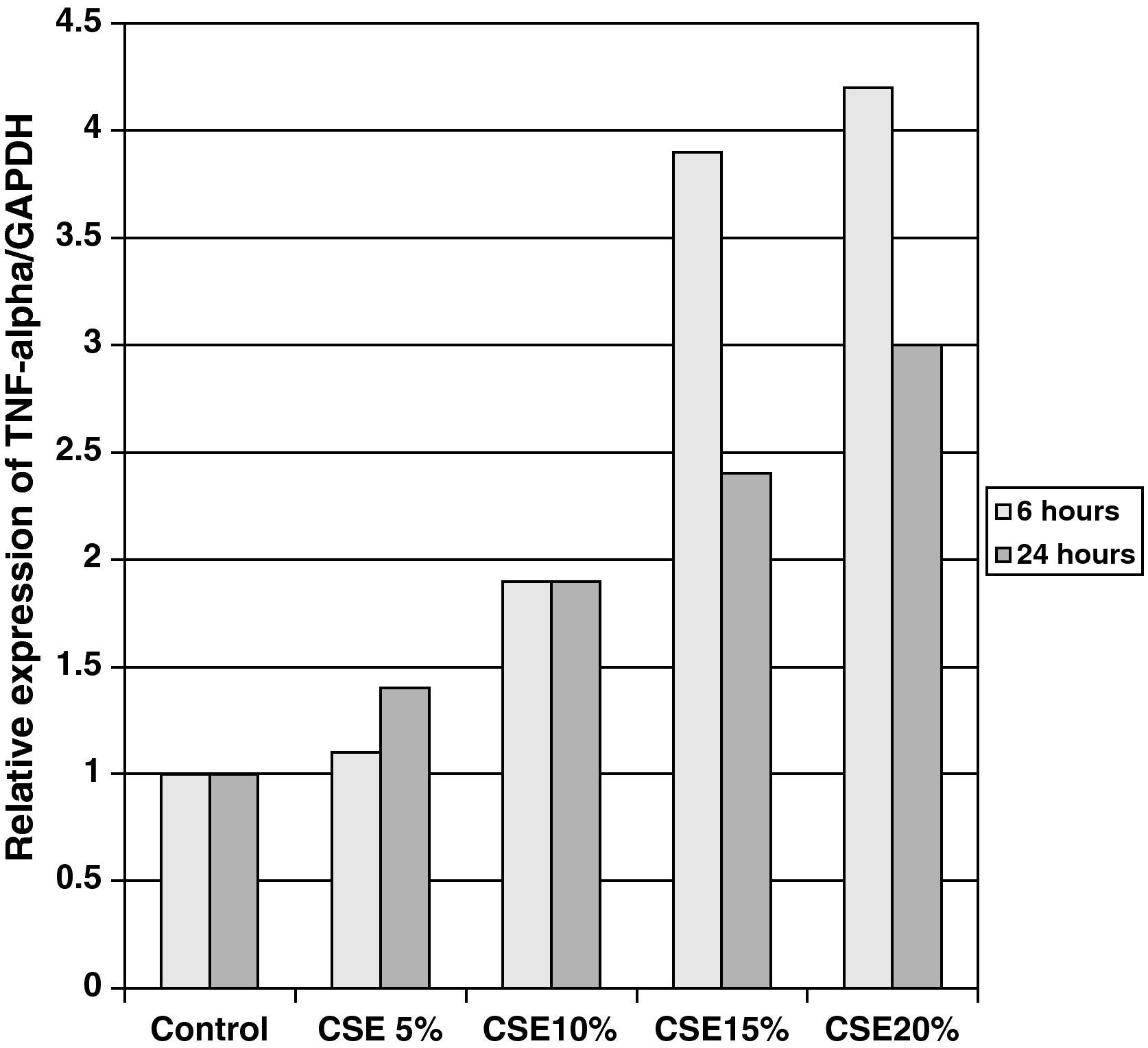
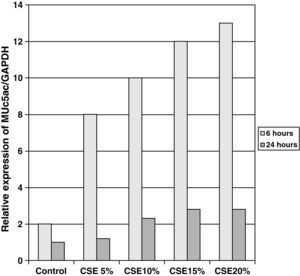
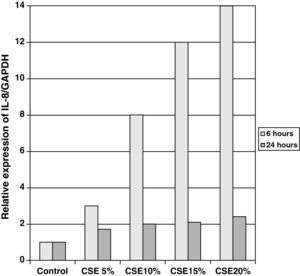
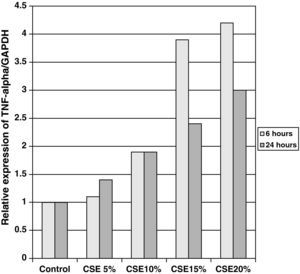
The samples obtained were cultured until differentiation was reached in pseudo-stratified epithelium with the presence of ciliated and calciform cells. Due to the difficulty entailed in the differentiation in an air–liquid interface (a mean of 40 days was needed per culture) and the limited material provided by the mucus biopsy, it was only possible to obtain viable cultures in 4 patients, all of whom were from the healthy smoker group. From each, 30 cultures were used for experimental analysis. The stimulation of the epithelial cells by the exposure to tobacco smoke produced a dose-dependent increase in gene expression obtained by means of RT-PCR of Muc5AC (10.3 [1.2]) (Fig. 1), IL8 (5.1 [0.3]) (Fig. 2), and TNFα (4.3 [0.4]) (Fig. 3) both in the 6h culture as well as after 24h (2.5 [0.3], 2.4 [0.2], and 2.9 [0.2]) compared to the baseline values. This effect in proportional terms was more intense in the 6h cultures and the expression fell progressively. In these experimental conditions, we did observe a significant correlation between the concentration of tobacco extract and the expression of IL8 (r=0.42; P<.05), TNFα (r=0.39; P<.05) and Muc5AC (r=0.47; P<.05). Some of these inserts were used to corroborate the epithelial differentiation with the presence of secretory and ciliary epithelial cells (Figs. 4 and 5).
There was also a significant correlation between the values of RNAm for IL8, TNFα, and Muc5AC.
DiscussionThe results of the study show that there are differences in the production and secretion of IL8 in the respiratory airways between healthy smokers and COPD patients. The higher the degree of obstruction, the higher the IL8 values; the values do not increase depending on the intensity or duration of tobacco habit. On the other hand, we have also been able to demonstrate that in differentiated bronchial epithelium cultures from smoker patients there is increased cell expression of Muc5AC, IL8, and TNFα. This indicates that, at least in the case of IL8, its elevation is due not only to the inflammation by neutrophils or macrophages, but also to a direct effect of the components of tobacco smoke on the epithelial cells.
The relationship between cigarette smoke and the pathogeny of COPD is multifactorial: increased recruitment of neutrophils and macrophages, release of cell mediators with oxidative capacity, inactivation of protease inhibitors and increase in the transcription of proinflammatory cytokines. The relationship between these factors and the fundamental findings that define the disease, at least in its initial stages, such as hypertrophy–hyperplasia of the secretory cells and mucus glands or the presence of lymphoid follicles of CD8 cells, has been the motivation for multiple hypotheses developed from in vivo as well as in vitro studies.
Since the studies by Keatings7 in 1996, it is known that the levels of IL8 and TNFα are high in the sputum of COPD patients and that this increase correlates with the intensity of neutrophilia. The difference between healthy smokers and COPD patients would be conditioned, according to this theory, by an increase in the chemotactic activity of IL8 on the neutrophils.22,23 This action would be increased in the case of other co-factors, such as the presence of infections or environmental contaminants. There is, however, dissociation in the studies published between the data from airway secretion samples24 and the histology findings in patients with incipient COPD in whom there is no observed neutrophilic inflammation of the mucosa but instead infiltration by mononuclear cells, made up of CD8+ lymphocytes that group together, forming lymphoid follicles.
Our study included COPD patients who had never been treated with inhaled anti-inflammatory medication, who as a group had a mild-moderate degree of obstruction and were free of bacterial infection. We found no differences in the neutrophilia of the secretions compared with healthy smokers, but we did observe an increase in the production of IL8 and TNFα, although only the former reached statistical significance. The lack of relationship between IL8 and the intensity of smoking or sputum neutrophilia (even though there was a relationship with the degree of obstruction) could be due to differences in the response pattern of other elements in the mucosa, such as the epithelial cells, regarding the production of mucus as well as the expression of cytokines. The lack of significant differences in TNFα values between both groups and the lack of correlation with clinical and functional parameters contrast with the data published in the literature, which indicate this mediator as a common point for the different alterations induced by tobacco. Our results from epithelial cultures corroborate this. It is possible that the differences in sample size or the lower severity of COPD compared with other studies explain these differences. As for IL13, although several studies25 in cells cultured in vitro have demonstrated their action in response to tobacco smoke, there are no comparative studies in airway secretions between healthy smokers with or without COPD; therefore, the absence of differences in our sample cannot be compared with other studies.
The absence of differences in the concentrations of NO is justified by the limited number of patients with bronchial hypersecretion compared with other studies.
In vitro studies of human epithelial cell cultures have provided much knowledge about the interactions between cytokines and mucins. Today we know that the exposure to tobacco smoke, both in animal models as well as in human cells, increases the expression of epidermal growth factor receptor (EGFR) that, at the same time, correlates with an increase in the release of TNFα, IL8, IL13, and mucins.
The mucins that constitute the most frequent glycoproteins in the mucus of the airways are produced by the goblet-type secretory cells as well as by the submucosa glands. In the former case, the most frequent mucin is Muc5AC, while in the glands the predominant secretion is Muc5B.13 In COPD patients, depending on the phenotype, there is an observed increase in Muc5B in patients with chronic bronchitis or an increase in Muc5AC in the studies of healthy smokers or in the initial phases of COPD. As explained in the introduction, the relationship between mucin production and tobacco smoke is produced as a consequence of the direct action of inflammatory mediators, such as TNFα or IL8, in the epithelial cells, favoring the glandular secretion or the release of neutrophilic elastase.
In our study, we have been able to observe that stimulation with incremental doses of cigarette smoke extract in the epithelial cell cultures of healthy smokers produced an increase in the expression of RNAm for TNFα and IL8, which correlated with the expression of Muc5AC.
In previous studies carried out in samples obtained from the bronchial biopsy of healthy smokers with or without COPD,26 differences have been demonstrated in the expression of Muc5AC in smoker subjects compared with non-smokers, but no differences were found with COPD patients. Nor were significant correlations observed between the expression of Muc5AC with the intensity of the neutrophilic inflammation or the severity of the obstruction. In studies about the culture of non-differentiated epithelial cells, tobacco smoke synergically increases the production of Muc5AC induced by EGFR, bacterial lipopolysaccharides or TNFα15 and their direct effect on the production of IL8.8 These studies, however, are not done in differentiated epithelial cultures or in COPD patients.
In this latter group, there are fewer studies. In a study in non-differentiated cultures of COPD patients,27 no differences were observed in the baseline expression of RNAm or production of IL8 compared with smokers without COPD, but they were observed after stimulation induced by TNFα. As was seen before, these facts corroborate that the neutrophils are not the inductors in the release of cytokines, but instead there are other mechanisms in which tobacco smoke or TNFα directly intervene in epithelial cells. In a similar study, but in this case done in differentiated epithelial cells of healthy ex-smokers and COPD patients, Patel et al.28 observed that, in baseline conditions, the production of IL6 and IL8 was not only unequal, but it was also lower in the COPD patients. Nevertheless, after stimulation with TNFα, a greater increase was observed in COPD patients.
According to the authors, the difference between the values obtained in secretion samples and the results in cell cultures from COPD patients is not attributable to the quality of the cultures (as they demonstrate their viability by showing ciliated cells) but instead to the greater inflammatory component that occurs in subjects in vivo.
In our opinion, this factor does not always hold true (as not all studies demonstrate differences in inflammatory pattern), but epithelial differentiation is important. The culture method for the differentiated epithelial cells by means of growth in an air–liquid interface (ALI) was developed in 1996 by Gray29 using commercial epithelial cell lines. In their study, they demonstrated that the characteristics of the medium, the number of passes and the presence of air in the apical segment allow for differentiation, not only of the ciliogenesis but also of the mucus-producing cells. The apical-basal differentiation is only possible by means of this air–liquid interface. Later studies30 have demonstrated that this method conditions the profile for transcriptional studies. Thus, the cluster profile of RNAm of different genes observed directly from biopsy samples is similar to that obtained in cultures of epithelial cells differentiated by ALI, both if they are tracheal or bronchial in origin, while they differentiate from the cultures done without this method.
The most reliable culture technique is, on the other hand, more laborious and the desired results are not always obtained.
In our case, by using samples taken by bronchial biopsy and not surgical pieces, the difficulty was even greater, and therefore we could only obtain results in healthy smokers. In this group and with direct exposure to tobacco smoke, we demonstrated that there is a dose-dependent increase in the production of mucin, secondary to the stimulation of secretory cells as the formation of glands is not possible, of IL8 and TNFα. The correlation between them shows that cigarette smoke alone is able to induce an inflammatory response in the epithelial cells of healthy smoker subjects. The decrease in RNAm expression after 24h, compared with levels after 6h, concurs with the transcriptional mechanisms of any inductor, having a peak action in the first few hours and decreasing at the same time the production of proteins increases, as has been demonstrated in other epithelial culture studies.
There are not many studies that use this technique in patient biopsies. In commercial cell line cultures,16 differentiation by means of ALI has shown that the production of IL8 and Muc5AC is only observed after stimulation with cigarette smoke at high concentrations (30%). The fact that in our study significant differences are observed with lower concentrations may indicate a greater sensitivity in the epithelium of smokers.
The fact that in the end we were only able to obtain viable cultures from smoker patients without COPD significantly limits the results and their generalized applicability to different COPD phenotypes. The translation of experimental research to daily clinical practice most certainly requires more extensive samples. Nevertheless, the finding that, at least in a significant number of well-differentiated inserts, the results concur with the hypothesis set forth can serve as a basis for further studies that correct the difficulties of this type of studies in bronchial mucosa biopsies.
In short, our study, with the inherent limitations of its difficulty, can conclude that there are differences in the production of cytokines between healthy smoker individuals and those with COPD that could be explained by the direct action of tobacco smoke on epithelial cells.
Please cite this article as: De Diego Damiá A, et al. Estudio del efecto de citocinas proinflamatorias en las células epiteliales de pacientes fumadores con o sin EPOC. Arch Bronconeumol. 2011;47:447–53.
Funded by the following grants: SVN 2004, SEPAR 2005, SAF2008-03113 and CIBERES (CB06/06/0027).