Eosinophilic pleural effusion (EPE), defined as pleural fluid with ≥10% eosinophils, comprises 10% of exudative pleural effusions, commonly associated with infections and malignancies, with only a limited number of drugs implicated.1
Venlafaxine, a serotonin–norepinephrine reuptake inhibitor (SNRI), is widely prescribed and is associated with several adverse effects. No prior reports link it to EPE.
This first documented case of venlafaxine-induced EPE with peripheral eosinophilia (PE) broadens known drug associations with this condition.
A 75-year-old male with osteoporosis, emphysema, and a 40-pack-year smoking history presented with dyspnea and pleuritic chest pain, one week after experiencing a fall. He denied fever, cough, hemoptysis, rash, or arthralgia and was not on any medication.
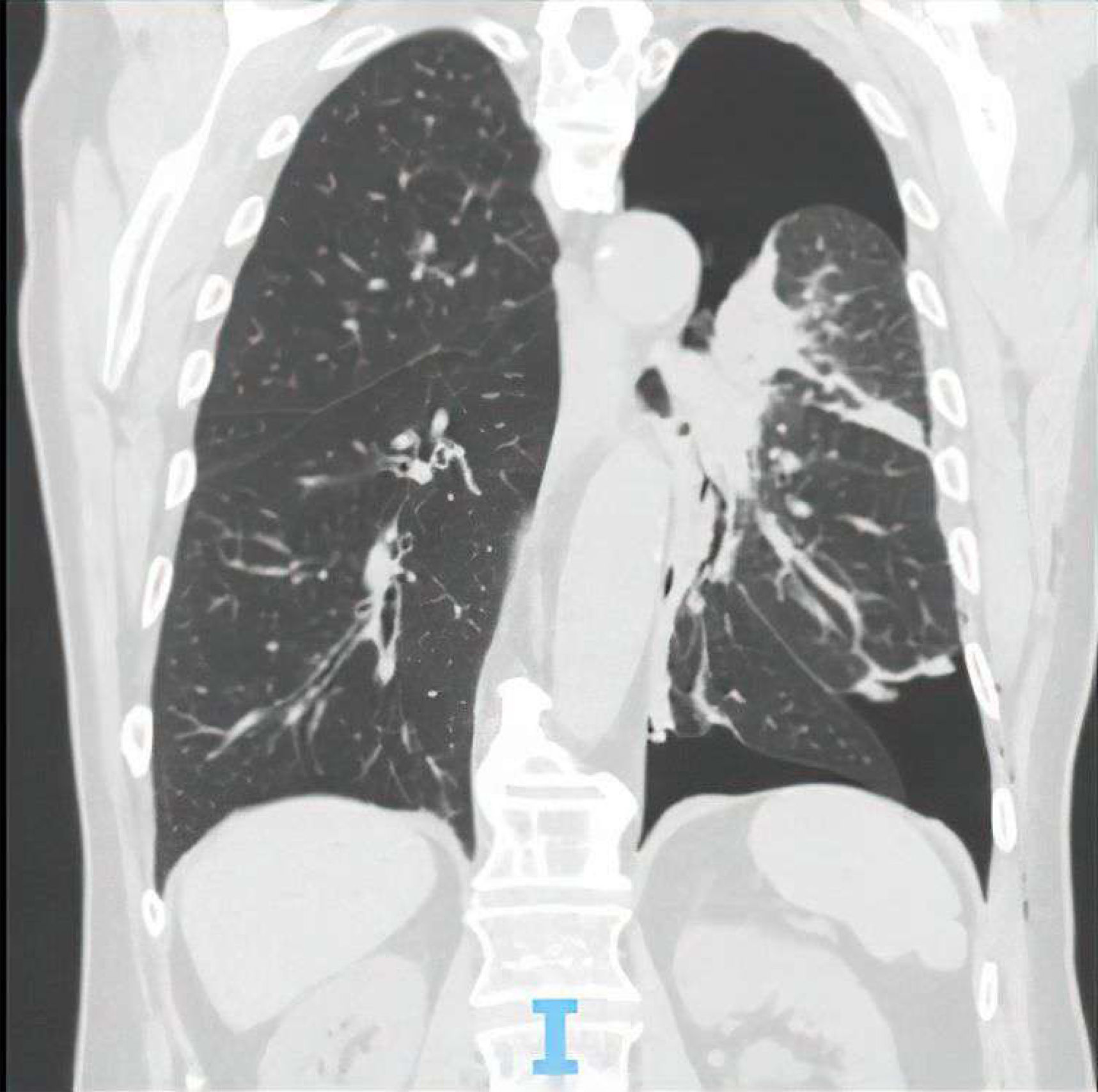
On admission, he was afebrile, eupneic, normotensive, and normocardic. Cardiopulmonary examination revealed diminished breath sounds and dullness over the left hemithorax. Arterial blood gas on room air showed hypoxemia (pO2: 69mmHg) with a normal pH. Laboratory results indicated normal hemoglobin, leukocyte, and eosinophil counts, with mildly elevated C-reactive protein (3.12mg/dL). Imaging revealed a hydropneumothorax and rib fractures not requiring surgery (Fig. 1). Chest drainage resolved the traumatic hydropneumothorax, with no residual pleural effusion or pneumothorax. Diagnostic thoracentesis was deferred due to procedural urgency.
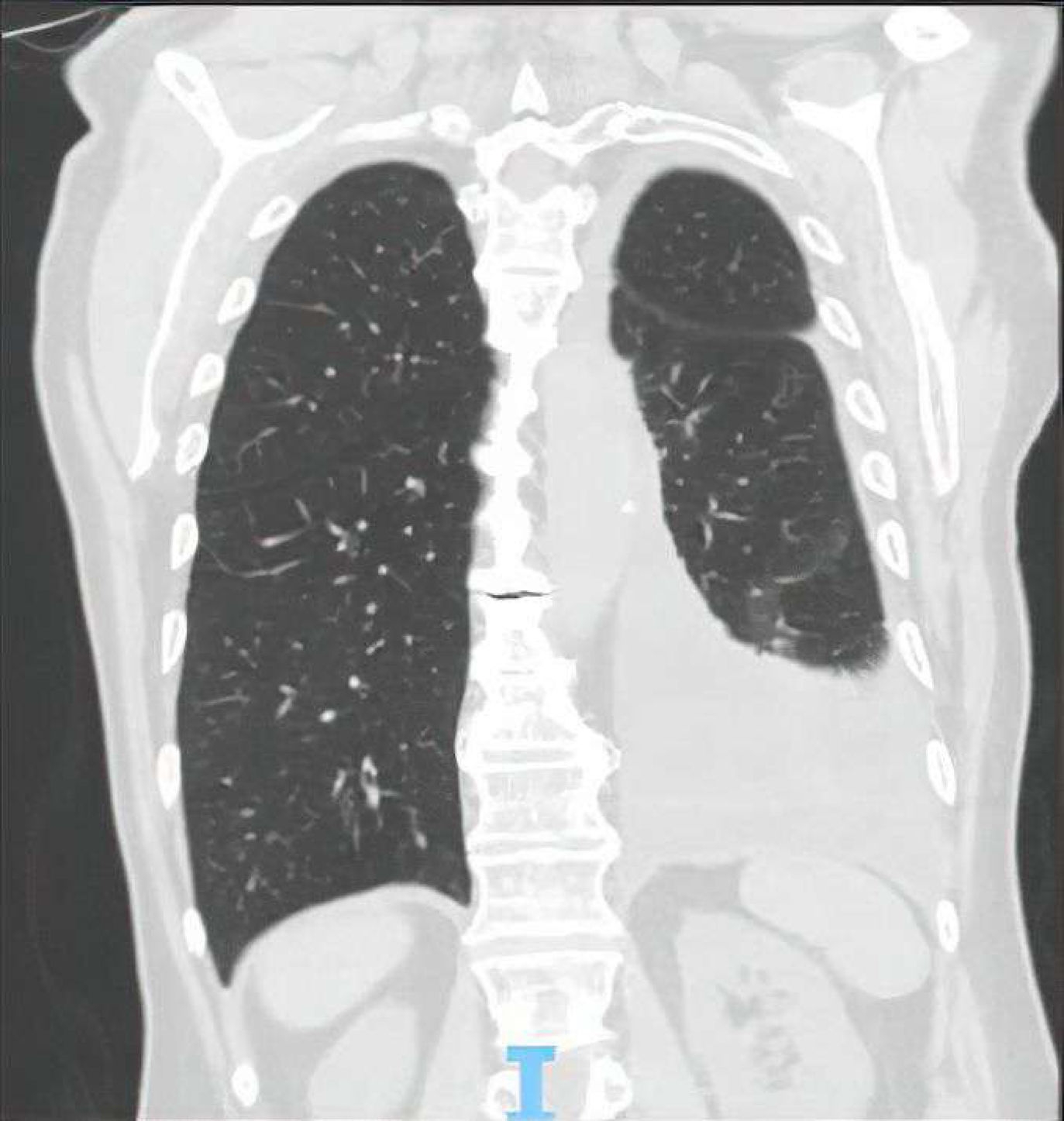
The patient remained hospitalized for unrelated reasons and developed generalized anxiety disorder, requiring venlafaxine therapy. Shortly after, he exhibited marked eosinophilia (45.2%), and a recurrent pleural effusion was evident 7 days later (Fig. 2). A thoracentesis drained 1200mL of yellowish fluid (pH 7.35) containing 52% eosinophils, consistent with an exudate per Light's criteria. Cytology showed no malignant cells, and pleural biopsies revealed nonspecific lymphoid aggregates. Cultures and parasitological tests were negative. Comprehensive investigations excluded neoplastic, autoimmune, or vasculitic etiologies. Excluding other etiologies suggested a drug-induced effusion, prompting sequential discontinuation of medication. Resolution followed venlafaxine withdrawal, though pain control and rehabilitation may have contributed. Venlafaxine-induced EPE and PE were ultimately assumed. The patient remained asymptomatic for over a year.
While pneumothorax can be associated with transient EPE,2 it had resolved before recurrence. Furthermore, PE, uncommon in pneumothorax-related effusions,2 its resolution after venlafaxine withdrawal, and EPE recurrence following PE onset further reinforce the drug's role. Re-exposure to venlafaxine was avoided due to recurrence risk and safer alternatives.
Drug-induced eosinophilic lung disease encompasses pulmonary eosinophilia, eosinophilic pneumonia, Churg-Strauss syndrome, and, rarely, EPE.3 Several drugs cause EPE,1,3,4 often through Type I or IV hypersensitivity.1,3 Venlafaxine has been reported in case series, predominantly linked to diffuse interstitial changes.5 EPE in this case likely stems from PE due to venlafaxine hypersensitivity, not previously reported.
Venlafaxine or its derived metabolites likely trigger a T-helper 2-cell-mediated response via interleukin-4 and interleukin-5 pathways,1,3,5 inducing eosinophil proliferation and PE development. Chemokines (eotaxin, RANTES [CCL5], MCP-3) likely drive eosinophil migration to the pleura.3 Cytokine receptor upregulation and eosinophil degranulation via IL-3, IL-5, and GM-CSF contribute to EPE-associated inflammation.2,3
This unique presentation highlights the need for vigilance in recognizing rare drug reactions. To our knowledge, this is the first documented case of venlafaxine-induced EPE, enriching understanding of its adverse effect profile and facilitating timely diagnosis and management.
Artificial Intelligence InvolvementThis manuscript underwent spell-checking and grammar refinement using artificial intelligence tools (ChatGPT) to enhance linguistic accuracy.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of InterestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.