The aim of this study was to assess several air-pressure settings for MI–E to determine their effect on peak cough flow (PCF), and to compare the best pressures with those are more common used in the literature (±40cmH2O) in patients with neuromuscular disorders (NMD).
MethodsAdults with NMD in whom MI–E was indicated were recruited. Assisted PCF was measured by an external pneumotachograph. The protocol included 9 PCF measures per patient: 1 baseline (non-assisted), 4 with increasing inspiratory pressures without negative pressure (10, 20, 30 and 40cmH2O or maximum tolerated), and then 4 adding expiratory pressures (−10, −20, −30 and −40cmH2O or maximum tolerated) with maximum inspiratory pressure previously achieved.
ResultsTwenty one patients were included, 61% with amyotrophic lateral sclerosis (ALS). Mean PCFs with recommended pressures (±40cmH2O) were lower than the scored in the individualized steps of the titration protocol (197.7±67l/min vs 214.2±60l/min, p<0.05). Regarding subgroups, mean PCFmax values in ALS patients with bulbar symptoms were significantly higher than those achieved with recommended pressures (163.6±80 vs 189±66l/min, p<0.05).
ConclusionThe PCFmax obtained with the protocol did not always match the recommended settings. It may be advisable to perform MI–E titration assessed by non-invasive PCF monitoring in patients with NMD, especially in ALS with bulbar involvement to improve the therapy detecting airway collapse induced by high pressures.
El objetivo del estudio fue evaluar varios ajustes de presión para insuflación-exuflación mecánica (IEM) para determinar su efecto sobre el flujo pico de tos (FPT), y comparar las mejores presiones con aquellas que son habitualmente utilizadas en la literatura (± 40 cmH2O) en pacientes con enfermedades neuromusculares (ENM).
MétodosSe reclutaron adultos con ENM en los que se indicó IEM. Se midió el FPT asistido mediante un neumotacógrafo externo, y se registraron las curvas de presión y flujo/tiempo. El protocolo incluía 9 medidas de FPT por paciente: una basal (no asistida), 4 con presiones inspiratorias crecientes sin presión negativa (10, 20, 30 y 40 cmH2O o máximo tolerado), y luego 4 presiones espiratorias (−10, −20, −30, −40 cmH2O o máxima tolerada) con la presión inspiratoria máxima conseguida.
ResultadosSe incluyeron 21 pacientes, 61% con esclerosis lateral amiotrófica (ELA). Las altas presiones (>±35cmH2O) lograron el mejor FPT solo en el 50% de los sujetos. Los FPT medios con presiones recomendadas de ±40 cmH2O (197,7±67l/min) fueron más bajos que los conseguidos con presiones de titulación individualizadas con el protocolo (214,2±60l/min, p <0,05). Con respecto a los subgrupos, los valores medios de FPT máximos en los pacientes con ELA con síntomas bulbares fueron significativamente mayores que los logrados con las presiones recomendadas (163,6±80 frente a 189±66l/min p <0,05)
ConclusiónEl FPT máximo obtenido con el protocolo no siempre coincide con las presiones más altas. Puede ser aconsejable realizar una valoración de IEM evaluada mediante monitorización no invasiva de FPT en pacientes con ENM, especialmente en ELA con afectación bulbar, para mejorar la terapia que detecta el colapso de la vía aérea inducida por altas presiones.
Patients with neuromuscular disease (NMD) are known to develop a restrictive pulmonary disease pattern due to progressive weakening of respiratory muscles.1 Other potential symptoms in the course of the disease are dysphagia, aspiration with recurrent respiratory infections; sleep disordered breathing, impaired cough and inability to clear secretions. Respiratory problems in general are the primary cause of morbidity and mortality in these patients.2,3
Inability to clear the airways of secretions due to ineffective cough is an important risk factor for acute respiratory failure in patients with NMD, and its association with infections and respiratory failure is the most common reason for hospitalization.3,4 Peak cough flow (PCF) is widely considered to predict cough efficiency.5,6 When PCF is below 160l/min, the cough is considered inefficient2,7 and, in this setting, the introduction of mechanical methods for assisting cough and preventing pneumonia, atelectasis or respiratory failure is recommended. Mechanical insufflation–exsufflation (MI–E) devices are used in patients with neuromuscular weakness to improve PCF, and to assist cough mechanically by applying positive and negative pressures to the airways, either non-invasively via a mask or invasively via tracheostomy.8–10
Despite the known benefits, there is no consensus regarding the optimal mechanical-assisted cough treatment regimen.11 While most European and American guidelines recommend the use of MI–E, the medical device industry does not provide reliable data to assist healthcare professionals in the decision-making process.12 Clinical studies have found insufflation and exsufflation pressures of up to 40cmH2O and −40cmH2O to be optimal in adults.13–15 Nevertheless, the risk of complications associated with high positive airway pressures, such as abdominal distension, worsening of gastroesophageal reflux, haemoptysis, chest and abdominal discomfort, acute cardiovascular effects and pneumothorax, should also be considered.10 Regarding therapy onset, there is not a standard starting model established. The procedure is currently performed by the respiratory therapist according to his/her criteria, applying the pressures recommended in the current literature as optimal (±40cmH2O). Tailoring treatment to ensure individual tolerance, while still optimizing cough effectiveness, is crucial.
The aim of this study was to assess several air-pressure settings for MI–E to determine their effect on PCF, and to compare the best pressures with those recommended in the literature as optimal in patients with NMD.
Materials and methodsAn observational prospective study was conducted in Parc Tauli University Hospital (Sabadell, Barcelona, Spain), with a catchment area of 400,000 inhabitants. Patients were considered for inclusion if they were diagnosed with NMD and presented a spontaneous PCF below 160l/min, which is the criterion for considering MI–E. Patients were recruited from the NMD multidisciplinary (Respiratory Medicine/Neurology) outpatient clinic.
Exclusion criteria were age <18 years, patients who had undergone tracheostomy, patients in an unstable medical condition or with psychiatric problems, and those with relative contraindications for MI–E (history of bullous emphysema, risk of pneumothorax or recent barotrauma). Patients with poor tolerance to MI–E, and intolerant to the first two steps of the protocol were also excluded (see below). The study protocol was approved by the Clinical Research Ethics Committee of Parc Tauli Sabadell Hospital (ref_2014/664), and written informed consent was obtained from all participants (ClinicalTrials.gov NCT03218215).
An ad hoc protocol was established to perform physiological assessment of the efficacy of MI–E. A Cough Assist T70 MI–E device® (Philips Respironics, Murrysville, Pennsylvania) was used for the study. Two respiratory therapists skilled in the in-exsufflation management performed the manoeuvres. The first one applied directly the therapy, using the “manual mode” (without fixed inspiratory and expiratory time) of the device. The second one was in charge of the monitoring procedures, controlling the quality of the tracings, reading the peak flow values, and storing them for later analysis.
The protocol was based on increasing steps of progressive pressure difference. Unassisted (baseline) PCF was first assessed, followed by incremental inspiratory and expiratory MI–E pressures (cmH2O) up to 80cmH2O maximum gradient (±40cmH2O, as recommended in the literature). An increase of 10cmH2O was made in each phase, starting with the inspiratory pressure (10–40cmH2O or maximum tolerated). Once the maximum inspiratory pressure tolerated by the patient was reached, the expiratory pressure was introduced following the same sequence (−10 to −40 cmH2O or maximum tolerated), keeping inspiratory pressure at the value previously considered as the most effective. When the maximum tolerated pressure gradient was lower than 80cmH2O (+40/−40) the PCF value achieved in this step was considered equivalent to the +40/−40cmH2O step. Treatment was considered to be non-effective if subjective tolerance was poor or the increase in assisted PCF manoeuvres was lower than 20% of the baseline PCF at the first two steps after three attempts.
The decision to switch to the next pressure step was based on the following criteria: haemodynamic stability, absence of desaturation measured by pulsoximetry, effectiveness of the previous pressure increase (increase in peak flow cough values) and good tolerance expressed orally by the patient. As in the first step, three attempts per step were tried before considering a pressure gradient level increase as non-effective, based on the following criteria: objective decrease of PCF, poor subjective tolerance of the patient after three attempts, oxygen desaturation or suddenly increase of heart rate more than 20%.
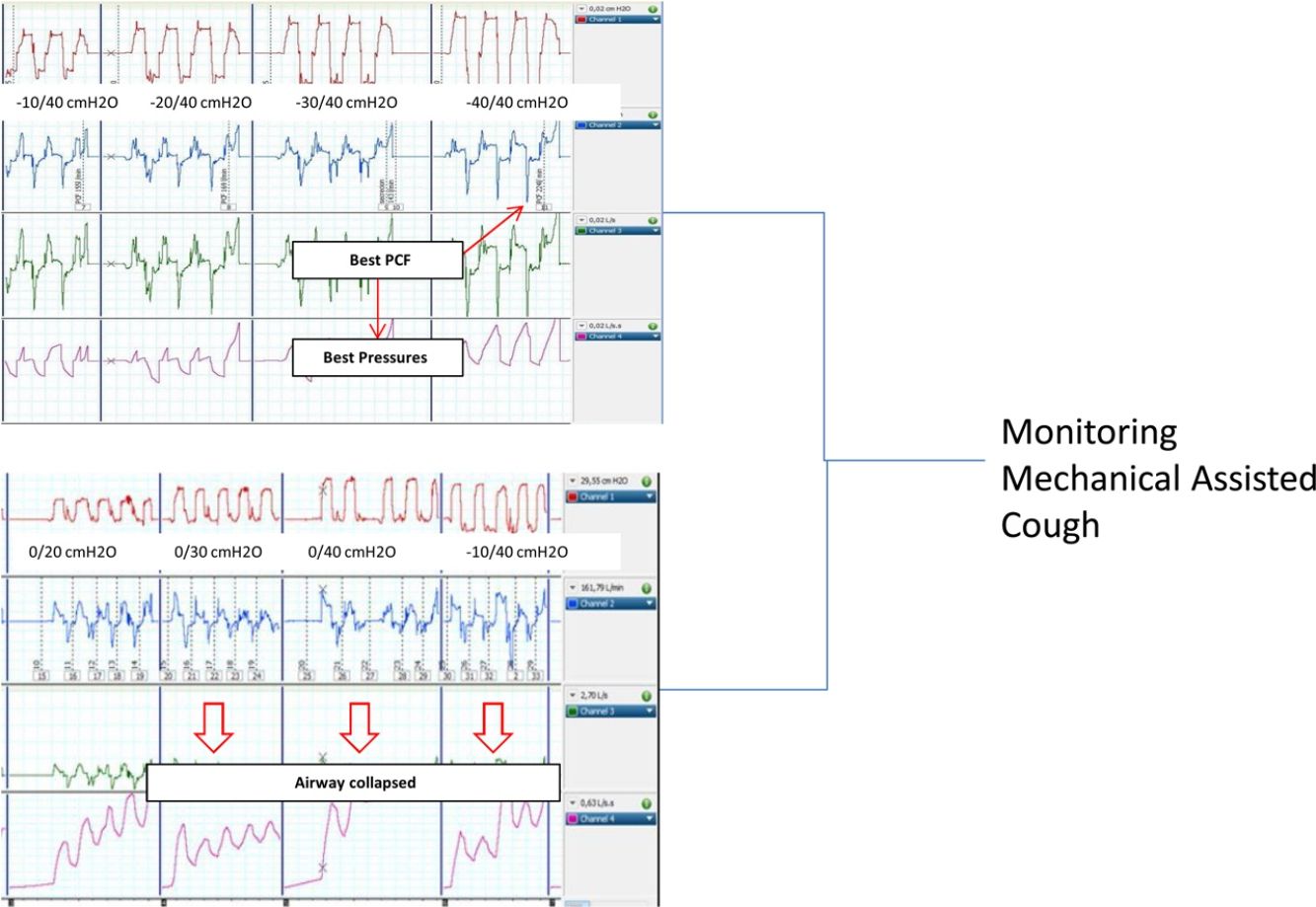
The best pressure combination was considered when the PCF was the highest achieved by the patient in this step (the highest value of three manoeuvres in each step) of the protocol (PCFmax) and the PCF achieved by recommended pressures was expressed as PCF±40cmH2O in the analysis.
As a signal acquisition system, an external polygraph (16Sp Powerlab, AD Instruments, Sydney, Australia) equipped with a pressure transducer (Model 1050) and a pneumotachograph (S300, AD Instruments, Sydney, Australia, with instrumental dead space 70mL, resistance 0.0018cmH2O/L/s) was connected to the MI–E device. Sampling frequency was set to 200Hz, and the polygraph was connected to a personal computer equipped with LabChart 7.0 software for Windows (AD Instruments, Sydney, Australia).
Waveform analysis to determine peak flow was performed automatically using peak analysis software (AD Instruments, Sydney, Australia). Tracings were visually reviewed to ensure the reliability of the peak flow automatic detection.
Since there are no recommendations in the interpretation of peak flow values measured externally with a pneumotachograph, a pre-use test was performed to determine the dynamic behaviour of the limb used in the study. Briefly, a compliance test was performed with increasing pressures and occlusion of the distal end of the tubing. As shown in Fig. 1a and b, the transition between inspiration and expiration was associated with a 100ms negative initial peak in flow waveform, corresponding to the compressible volume. Based on these findings, true PCF measurement values were considered reliable if the peak width was >100ms; if not, it could eventually correspond to the compressible volume inside the limb during the I–E transition. Up to three I–E manoeuvres were performed per step, and the highest PCF among them was considered as the optimal PCF.
Compressible volume identification in flow waveform, through a pre use test (see text for more details). (a) A clinical tracing of a patient, demonstrating a double peak in expiratory flow waveform. (b) The results of a pre-use test to determine semiology associated with compressible volume, which produces an initial 100ms peak. This peak was systematically ruled out in the PCF estimation (e.g., in (a), the second peak, labelled as PCF represents the true PCF).
Oxygen saturation (SpO2%) and heart rate (beats per minute, bpm) before and after each phase were also recorded to monitor patient status. Patients with amyotrophic lateral sclerosis (ALS) were assessed using the ALS Functional Rating Scale – revised (ALSFRS-r).16 The bulbar impairment score was evaluated from the ALSFRS-r, from where the items of speech and swallowing were calculated. A score higher than 6 in these items suggested bulbar involvement.
All variables were screened for normality by means of the Shapiro–Wilk test. Demographic and quantitative values were expressed as mean±standard deviation when the distribution was normal and median [interquartile range (IQR)] if not. Quantitative values were compared by means of paired Student's t tests or by the corresponding non-parametric test (Wilcoxon signed-rank test) in case of non normal distribution. The differences of PCFs between subgroups were assessed by the Mann–Whitney U test. To compare PCF achieved between phases and subjects with the MI–E protocol, the general model for repeated measures was used. When the sphericity of the model could not be assumed (Mauchly's test with p-value <0.05), the significance of the model was assessed with the Greenhouse–Geisser correction. The significance level was set at p-value <0.05 for all tests used. Statistical analyses were performed using IBM SPSS Statistics 23 for Windows (SPSS Inc., Chicago, IL, USA).
ResultsTwenty-five patients with NMD were screened for the study; 21 patients (13 men) were eventually confirmed eligible and included. The remaining 4 patients were totally clinical intolerant to the treatment even at the first step of the protocol. Three patients did not complete the entire protocol due to decrease of cough peak flow at increasing pressures. In this sample, 66.7% of patients (14 out of 21) had ALS and 33.3% had another NMD. In the ALS group, 7 out of 14 patients (50%) had bulbar involvement. Anthropometric and clinical values are summarized in Table 1. Median PCF measured at baseline was 57 [38–102]l/min, with no significant differences between ALS and non-ALS patients.
Clinical and anthropometric data of the patients included (n=21).
| All cohort | Bulbar ALS | Non-bulbar ALS | Dystrophies | Other NMDs | |
|---|---|---|---|---|---|
| Age (years) (mean, SD) | 63.9±15.75 | 70.7±14.6 | 71±7.8 | 49±19 | 50.6±6 |
| PCF baseline (l/min) (median, IQR) | 57 [38.3, 102.5] | 57.4 [21.5, 129.5] | 44 [36.7, 7] | 74.8 [30.3, 104] | 95 [57, 131] |
| SpO2 baseline (%) (mean, SD) | 95.4±1.7 | 96±1 | 94.4±1.8 | 95.7±2.2 | 96.3±1.5 |
| SpO2 post (%) (mean, SD) | 95±2.4 | 96.5±2.3 | 93.8±1.3 | 93.6±3.5 | 96±2.6 |
| BMI (kg/m2) (mean, SD) | 24±5 | 20.7±2.7 | 23.7±5 | 27±6.5 | 26.3±3.5 |
| HR baseline (bpm) (mean, SD) | 88±17 | 86±17 | 91±14 | 87±25 | 84±12 |
| HR post (bpm) (mean, SD) | 89±16 | 90.75±17 | 88±15 | 93±18 | 87±15 |
| ALSFR scale (score) (mean, SD) | NA | 21.3±10.4 | NA | NA | NA |
ALSFR scale: amyotrophic lateral sclerosis functional rating scale; BMI: body mass index; HR: heart rate (bpm); PCF: peak cough flow; SpO2: oxygen saturation; SD: standard deviation; IQR: interquartile range; NA: not applicable.
The included patients did not present any complications, like clinical distress or desaturations in the pulse oximetry during the titration process. Statistically significant differences were found for the entire cohort and all the subgroups in the comparison between PCFbaseline and PCFmax (74.6±49.7 vs 214±59l/min, p<0.001).
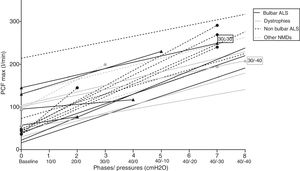
Interestingly, significant differences were found between PCF±40cmH2O reached with recommended pressures and the PCF max achieved in the individualized steps of the titration protocol (197.6±67 vs 214±59l/min, p<0.05). In the stratified analysis for baseline disease, statically significant differences in the achieved PCFmax in patients with bulbar ALS compared with the other two subgroups were found (see Table 2). As a result, 8 out of 21 patients (38%) achieved the best PCF with recommended MI–E pressures (±40 cmH2O). Finally, significant differences were found (p<0.05, Mann–Whitney U test) in PCFmax values in patients with and without bulbar symptoms (189±66l/min and 251±56l/min, respectively), but, no significant differences between them in PCF±40cmH2O (p<0.05, Mann–Whitney U test, 164±80 and 243±80l/min, respectively).
Repeated measures analysis showed that, generally, increasing MI–E pressures increased PCF in the subjects in a quasi-linear pattern, with significant differences (p<0.01, general linear model for repeated measures). Individual PCF and information about the step where PCF max was achieved is reflected in Fig. 2.
DiscussionThe most relevant findings of the present study are that the MI–E pressures considered in the literature as optimal17–19 are not always the most appropriate for the patient to achieve the highest assisted PCF (Table 3). PCFs in our study were mostly achieved with protocol pressures, and were higher than those obtained with the recommended pressures. In this setting, PCFs reached in intermediate steps in the study protocol would be more effective in up to 62% of cases than PCFs obtained with the recommended MI–E pressure. This difference is significantly higher in ALS patients with bulbar involvement. In fact, in these patients, the PCF±40cmH2O, values were in the range of mechanical assisted cough indication (around 160l/min).
Combination pressures achieving maximum peak inspiratory flow and diagnosis for each patient included in the study.
| Patient | Inspiratory pressure (cmH2O) | Expiratory pressure (cmH2O) | Diagnosis |
|---|---|---|---|
| P01 | 40 | −40 | SMD |
| P02 | 30 | −30 | ALS |
| P03 | 40 | −40 | ALS |
| P04 | 30 | 0 | FA |
| P05 | 40 | −10 | SMD |
| P06 | 40 | −40 | SMD |
| P07 | 40 | −10 | ALS |
| P08 | 40 | −40 | ALS |
| P09 | 30 | −40 | DMD |
| P10 | 40 | −30 | CMTD |
| P11 | 40 | −40 | ALS |
| P12 | 40 | −40 | ALS |
| P13 | 40 | −40 | ALS |
| P14 | 20 | 0 | ALS |
| P15 | 40 | 0 | ALS |
| P16 | 40 | −30 | CMTD |
| P17 | 40 | −30 | ALS |
| P18 | 20 | 0 | ALS |
| P19 | 40 | −40 | ALS |
| P20 | 40 | −30 | ALS |
| P21 | 40 | −30 | ALS |
ALS: amyotrophic lateral sclerosis; SMD: Steinert myotonic dystrophy; DMD: Duchenne muscular dystrophy; FA: Friedreich's ataxia; CMTD: Charcot-Marie-Tooth disease.
It would be questionable the clinical meaning of such differences (around 30l/min in cough peak flow), but if we consider that the conditions of the pulmonary and chest wall mechanics do not change between the steps of the protocol, the lack of response in PCF at increasing pressures suggests that the patient presents some kind of intolerance to this pressure increase (upper airway collapse, muscular effort against external pressures, and so on). This intolerance may eventually lead to poor compliance, lack of clinical benefit or even to undesired side effects of the technique.
Since cough effectiveness is correlated with PCF,20 it seems important to optimize combinations to avoid excessive pressure values that may eventually lead to poor compliance and complications during therapy.
Three phases are needed for a normal cough: inspiration, compression and expulsion. During the inspiration phase, healthy subjects achieve intrapulmonary volumes of 2.3±0.5L to obtain optimal PCFs.21 This phase needs a maximal inspiration (conserved diaphragmatic function) until 60%–80% of the total lung capacity is reached, with the higher inspiratory volumes being linked to a more effective cough.5,6,22 In the compression phase, the glottis closes during a 0.2s interval to prevent any outflow of inhaled air, and finally the glottis opens, usually by recruiting expiratory (abdominal) muscles in the expulsion phase.21,23,24
Patients with NMD can present upper airway collapse, especially during therapies with positive or positive–negative external pressure devices (non-invasive ventilation or MI–E), either as a reflection of high inspiratory volume or by collapsing hypotonic anatomical upper airway structures. If an upper airway event occurs, the effectiveness of MI–E is reduced, and in extreme situations is completely ineffective.
The pressure range required to achieve a high expiratory flow mimicking cough is known to be between 15 and 45cmH2O.3 Current guidelines recommend the use of MI–E in order to improve clearance of airway secretions,19,23,25,26 but fail to provide recommendations on frequency or intensity.20 Some groups have suggested that pressures be determined individually to prevent side effects, but this is performed based on experience or the patient's tolerance, without providing an objective parameter for individualizing the pressure combination.20,27 Nevertheless, it is commonly accepted that the treatment is not always well tolerated, especially in advanced ALS with bulbar symptoms. These patients usually do not tolerate MI–E because the flow generated by the device induces a glottic narrowing or even closure during the expiratory phase.28,29
A recent study described laryngeal response patterns during the MI–E, assessed by transnasal fibre-optic laryngoscopy in patients with ALS. Results suggested that adduction of primarily supraglottic laryngeal structures during insufflations may be critical, and may compromise the effectiveness of MI–E in patients with ALS. This study also suggests that an individual approach could be important. Finally, lower inspiratory pressures and airflow values combined with longer inspiratory time may contribute to better laryngeal stability during insufflations, by reducing the impact induced by the Bernoulli effect.30 In the case of treatment failure, the authors propose laryngoscope assessment. In this setting, non-invasive assessment by measuring PCF with an external pneumotachograph, as proposed in the present study, could be a valid alternative to detect collapsed upper airway by observing any decrease in the peak flow between steps of pressure increase.
The study presents a limitation inherent to the pathology of our patients. NMDs present a progressive course, particularly ALS; it is thus possible that optimal pressure combinations may change over time during the course of the disease or show short-term variability. It seems recommendable the periodic reassessment of these pressures, especially in patients with rapid decline of pulmonary function or in those uncomfortable with the MI–E device. In a similar way, patients were recruited during the adaptation process, so that tolerance may increase after several days/weeks of use, requiring reassessment. Regarding technical procedure, the protocol increase order caused that certain pressure combinations were less likely than others (for example 10/−10, 20/−20 and so on) and interpretation of waveforms during a cough manoeuvre is not standardized. Finally, the complexity of the procedure (two respiratory therapists, need for external calibrated monitoring) makes the protocol difficult to perform in the daily practice.
In conclusion, the PCFmax obtained did not always match the PCF achieved with recommended combination pressures (PCF±40cmH2O). It seems advisable to titrate MI–E pressure combinations assessed by PCF monitoring in patients with NMD to improve effectiveness, especially in patient with bulbar ALS. Finally, monitoring PCF non-invasively with a pneumotachograph could be an alternative to endoscopic procedures for detecting upper airway collapse due to MI–E and finally to determine the real efficacy of the therapy.
FundingThis work was supported by a grant of Catalan Society of Respiratory Medicine (SOCAP) and Spanish Society of Pneumology and Thoracic Surgery (SEPAR).
Conflict of interestsThe authors declare that they have no conflict of interests.