Human evolution represents one of the most fascinating scientific and social challenges. Understanding how we came to acquire our current anatomical configuration is not only an academic pursuit but also essential for interpreting aspects of our biological and cultural identity. Over the last four million years, our evolutionary history has progressed from primitive, tree-dwelling primates to modern humans through successive species belonging to two major genera: Australopithecus and Homo (Fig. 1A).
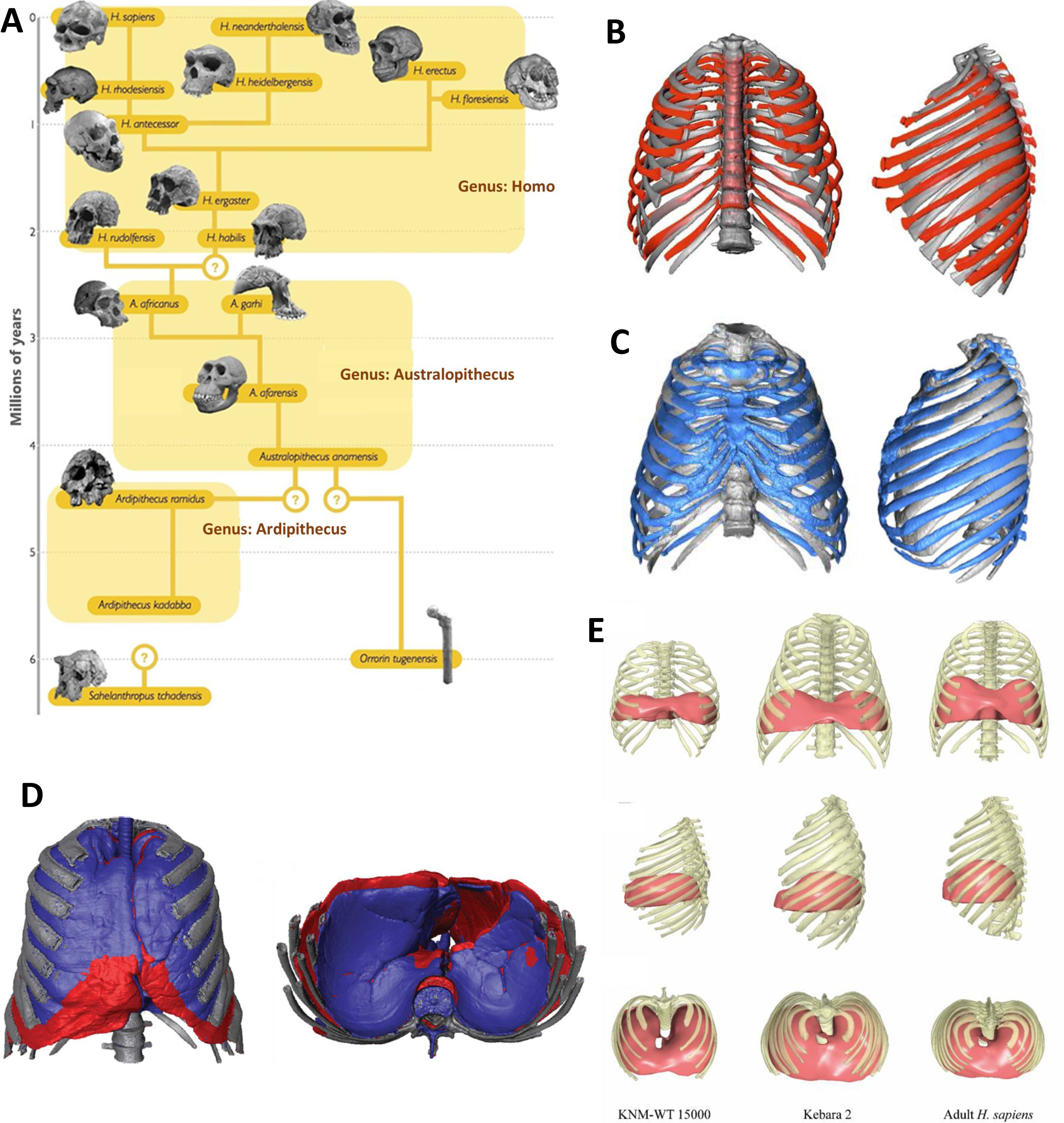
Morphological changes in the thorax throughout human evolution. (A) Schematic representation of the evolution of the genera Australopithecus and Homo, including some of their most representative species. (B) Superimposition of the 3D reconstruction of an adult H. ergaster thorax (KNM-WT 15,000, in red) onto that of a modern adult human using Procrustes registration. H. ergaster retains a conical-shaped thorax, characterized by a narrower upper portion and a broader and deeper lower portion. Adapted from Bastir et al. [12]. (C) Superposition of the Neandertal thorax (Kebara 2 male, in blue) and a modern human male specimen (in gray) in ventral and left lateral views. Although relatively similar in the upper hemithorax, the Neandertal ribcage is wider and deeper in its lower portion. Adapted from Gómez-Olivencia et al. [13]. (D) Superimposition in frontal and caudal views of Neandertal (in red) and modern human (in blue) ribcages and lung shapes. Adapted from García-Martínez et al. [15]. (E) Frontal, lateral, and cranial views of reconstructed ribcages and upper diaphragms of Homo ergaster (KNM-WT 15,000) and Neandertal (Kebara 2) specimens compared to the mean ribcage and upper diaphragm of an adult Homo sapiens reference sample. Adapted from López-Rey et al. [20].
Among the many factors that shaped this evolutionary trajectory, the acquisition of upright posture and bipedalism stands out as one of the most decisive milestones. Walking upright profoundly transformed our anatomy, physiology, and behavior, ultimately paving the way for the emergence of the genus Homo. While pelvic and limb adaptations to bipedal locomotion have been widely studied, the thoracic cage—central to respiration and metabolic performance—has received comparatively less attention despite its evolutionary significance.
In quadrupeds, respiratory biomechanics are tightly coupled to locomotion and thermoregulation through panting [1]. The forward and backward displacement of abdominal contents during running directly affects diaphragm motion, such that one locomotor cycle corresponds to a single breathing cycle. This limits endurance capabilities. In contrast, bipedalism decoupled breathing from gait cycles, while thermoregulation shifted to sweating. This independence provided early Homo with a more efficient respiratory system, granting an evolutionary advantage in open savanna environments by enabling endurance activities and new subsistence strategies based on animal protein that fueled both body and brain evolution [1].
The study of the thorax is crucial because pulmonary ventilation varies greatly depending on posture. In modern humans, distinct ventilatory patterns are observed in upright versus prone positions, suggesting that the shift to habitual bipedalism imposed new mechanical demands on thoracic organization [2,3]. Our relatively flat, cylindrical thorax, with curved, declined ribs and a tall, narrow waist, allows excellent diaphragmatic mobility, powerful expiration, and ribcage flexibility. These traits provide a highly efficient respiratory system adapted for sustained activity and endurance locomotion. Analyzing the ontogeny and phylogeny of the thorax offers valuable insights into how these functional advantages evolved [4,5].
The earliest bipeds, represented by Australopithecus, appeared about four million years ago. Australopithecus anamensis (4.2–3.9million years ago [Ma]) combined primitive traits associated with climbing with adaptations for facultative bipedalism. Its thoracic cage was likely conical—narrow at the top and wide at the base—similar to modern great apes, indicating limited ventilatory capacity, especially during sustained exertion [6]. Australopithecus afarensis (3.9–2.9Ma), exemplified by the famous fossil “Lucy,” displayed a skeleton more clearly adapted to upright walking, yet it retained a conical thorax consistent with a less efficient breathing pattern than modern humans [7]. Later, Australopithecus africanus (3.0–2.0Ma) showed a somewhat more gracile morphology with reduced sexual dimorphism and a rounder cranial vault, but its thoracic cage likely maintained the same primitive structure.
A conical thorax has specific functional implications. The narrow upper region favors upper limb mobility, advantageous in a partially arboreal lifestyle, but it restricts pulmonary expansion. The wide, deep lower region, combined with low rib torsion, limits thoracic flexibility and diaphragmatic efficiency [8]. Together, these traits indicate that the evolution toward a modern, endurance-optimized thorax was still incomplete [9].
In modern humans, thoracic ontogeny reflects a dynamic, functionally driven process. During growth, the upper thorax expands markedly and becomes distinct from the lower region, which increases less and narrows in relative transverse dimensions. Anatomical and imaging studies, from newborns to adults, document this transformation, which optimizes thoracic space for diaphragmatic function and ventilatory efficiency [10,11]. The adult cylindrical thorax of Homo sapiens represents the culmination of this developmental and evolutionary pathway.
The earliest Homo species, such as Homo habilis and Homo rudolfensis (2.5–1.6Ma), are sometimes referred to as transitional forms. They retained many australopithecine features, including body sizes of roughly 130cm and 40kg, and primitive bipedal traits. However, their cranial volume increased substantially, reflecting higher energy requirements. Their thoracic morphology was probably still similar to that of australopithecines, implying limited respiratory performance.
A major evolutionary step occurred with Homo ergaster (1.8–1.5Ma), often considered the African form of Homo erectus. These hominins were taller and heavier—adult males reaching up to 80kg—with robust builds, wide hips, and reduced sexual dimorphism. Their cranial capacity ranged between 850 and 950cm3, and they displayed thinner cranial bones, smaller faces, and prominent supraorbital tori, although they lacked a high forehead or chin. The reduction of molars and premolars suggests a diet rich in animal protein. It has been proposed that H. ergaster may have developed early forms of articulate speech, possibly related to cooperative hunting or scavenging.
The well-preserved Turkana boy skeleton, belonging to an 8–9-year-old who lived 1.6Ma near Lake Turkana, provides crucial information about this species. Three-dimensional reconstructions reveal a thorax considerably larger than that of australopithecines but still narrow superiorly and very wide and deep inferiorly (Fig. 1B) [12]. This primitive organization indicates that the thoracic configuration typical of modern humans had not yet evolved, probably reflecting a more massive overall body structure.
Homo neanderthalensis, who inhabited Europe and western Asia between approximately 230,000 and 29,000 years ago, represents an intermediate stage in thoracic evolution. Adapted to cold climates, Neanderthals had short, stocky, muscular bodies with relatively short limbs and broad chests to conserve heat. Their cranial capacity averaged about 1500cm3, slightly larger than that of modern humans, and their skeletons showed wide pelvises, robust bones, and mandibles without chins, with characteristic retromolar gaps. Thoracic reconstructions reveal a distinctive morphology: while the upper thorax resembled that of modern humans, the lower thorax expanded markedly in the transverse plane (Fig. 1C), corresponding to their broad pelvis [13,14]. This configuration yielded very high total lung capacity estimates (Fig. 1D) [15]. Initially, this might seem advantageous, but their low degree of rib torsion and deep caudal thorax reduced thoracic mobility and diaphragmatic efficiency. As a result, ventilatory mechanics were less economical, limiting aerobic endurance compared with H. sapiens (Fig. 1E) [15–17].
Modern humans, who emerged around 300,000 years ago, reached the peak of thoracic evolution. Our flatter thorax, curved and declined ribs, and narrower lower hemithorax optimize mobility and diaphragmatic function [12,18]. These adaptations likely provided an edge for persistence hunting and long-distance travel, vital in open environments such as savannas.
During the period of overlap between Neanderthals and modern humans (45,000–24,000 years ago), Europe experienced dramatic climatic shifts, including glacial phases that transformed ecosystems. The reasons for Neanderthal extinction remain debated and may include violent conflict, disease, environmental change, or inbreeding. A further possibility is that their less efficient ventilatory mechanics limited adaptability in demanding environments [19,20]. In contrast, H. sapiens, with superior endurance and energy economy, may have better supported the metabolic costs of long-distance travel, cooperative behavior, and brain expansion. While likely not the sole cause, this physiological edge may have helped tip the balance toward our species.
In conclusion, the evolutionary history of the thoracic cage reflects the intertwined demands of locomotion, respiration, and metabolism triggered by bipedalism. Early hominins retained a primitive conical thorax, whereas later species progressively developed a cylindrical, endurance-optimized structure. Neanderthals, despite their robust builds and large thoracic volumes, did not complete this transition. H. sapiens represents the culmination of this process, and its thoracic adaptations may have been decisive for our ecological dominance and global success.
DedicationTo Maribel Torres, a teacher, a friend, and a vital part of all we do.
Contribution of each authorMB and FGR contributed equally to the conceptualization and methodology of the study, writing the original draft of the manuscript, reviewing and editing it, and creating the figure. Both authors have full access to all the data in the study and had final responsibility for the decision to submit for publication.
Artificial intelligence involvementArtificial intelligence tools were not used in the preparation of this manuscript.
FundingNone.
Conflict of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.


![Morphological changes in the thorax throughout human evolution. (A) Schematic representation of the evolution of the genera Australopithecus and Homo, including some of their most representative species. (B) Superimposition of the 3D reconstruction of an adult H. ergaster thorax (KNM-WT 15,000, in red) onto that of a modern adult human using Procrustes registration. H. ergaster retains a conical-shaped thorax, characterized by a narrower upper portion and a broader and deeper lower portion. Adapted from Bastir et al. [12]. (C) Superposition of the Neandertal thorax (Kebara 2 male, in blue) and a modern human male specimen (in gray) in ventral and left lateral views. Although relatively similar in the upper hemithorax, the Neandertal ribcage is wider and deeper in its lower portion. Adapted from Gómez-Olivencia et al. [13]. (D) Superimposition in frontal and caudal views of Neandertal (in red) and modern human (in blue) ribcages and lung shapes. Adapted from García-Martínez et al. [15]. (E) Frontal, lateral, and cranial views of reconstructed ribcages and upper diaphragms of Homo ergaster (KNM-WT 15,000) and Neandertal (Kebara 2) specimens compared to the mean ribcage and upper diaphragm of an adult Homo sapiens reference sample. Adapted from López-Rey et al. [20]. Morphological changes in the thorax throughout human evolution. (A) Schematic representation of the evolution of the genera Australopithecus and Homo, including some of their most representative species. (B) Superimposition of the 3D reconstruction of an adult H. ergaster thorax (KNM-WT 15,000, in red) onto that of a modern adult human using Procrustes registration. H. ergaster retains a conical-shaped thorax, characterized by a narrower upper portion and a broader and deeper lower portion. Adapted from Bastir et al. [12]. (C) Superposition of the Neandertal thorax (Kebara 2 male, in blue) and a modern human male specimen (in gray) in ventral and left lateral views. Although relatively similar in the upper hemithorax, the Neandertal ribcage is wider and deeper in its lower portion. Adapted from Gómez-Olivencia et al. [13]. (D) Superimposition in frontal and caudal views of Neandertal (in red) and modern human (in blue) ribcages and lung shapes. Adapted from García-Martínez et al. [15]. (E) Frontal, lateral, and cranial views of reconstructed ribcages and upper diaphragms of Homo ergaster (KNM-WT 15,000) and Neandertal (Kebara 2) specimens compared to the mean ribcage and upper diaphragm of an adult Homo sapiens reference sample. Adapted from López-Rey et al. [20].](https://static.elsevier.es/multimedia/03002896/unassign/S0300289625003333/v1_202510150424/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)







