Bronchiectasis represents in frequency the third chronic inflammatory airway disease after chronic obstructive pulmonary disease (COPD) and asthma. It is produced by more than one hundred causes, both pulmonary and extrapulmonary. Despite advances in recent years in the understanding of this condition and the publication of several national and international guidelines on its management, in most cases the etiology remains unknown. Among the identified etiological forms, post-infectious and post-tuberculous are the most frequent. It is also striking how bronchiectasis associated with COPD and severe asthma has been progressively increasing over the years, probably due to greater awareness among healthcare professionals of the importance of such associations and the wider use of chest computed tomography (the diagnostic method of choice for bronchiectasis from a radiological perspective). However, it is remarkable, according to data obtained from national and international bronchiectasis registries, the considerable geographic heterogeneity in their etiology. Thus, in socially disadvantaged regions or in those with poorer healthcare access, post-infectious and particularly post-tuberculous forms clearly predominate. It is always necessary to perform the appropriate complementary tests, as highlighted in all bronchiectasis guidelines, to exclude at least the treatable etiologies (treatable trait), since this is undoubtedly associated with a better patient prognosis.
Bronchiectasis in adults is currently recognized as the third most prevalent chronic inflammatory airway diseases, following asthma and chronic obstructive pulmonary disease (COPD), and is often closely associated with the more severe forms of these latter two conditions [1,2]. Radiologically, bronchiectasis is characterized by a dilation of the airway lumen; pathophysiologically, it involves chronic inflammation, typically of mixed cellularity with a predominance of neutrophils [3]; and clinically, it manifests as chronic cough, usually accompanied by increased sputum volume, density, and/or purulence, as well as recurrent exacerbations of an infectious profile throughout its natural history [4]. Proteolytic and pro-inflammatory products derived from bronchial inflammation and infecting microorganisms most commonly pathogenic bacteria, lead to progressive destruction of the bronchial wall, culminating in the characteristic airway lumen enlargement [3]. This establishes a vicious vortex driven by the synergistic interaction of inflammation and infection, each potentiating the other [5].
Despite similarities in pathophysiology and radiographic findings, bronchiectasis arises from a wide array of causes, both intrapulmonary and extrapulmonary, and may be linked to past or present conditions [6,7]. Of all etiologies, cystic fibrosis (CF) and primary ciliary dyskinesia (PCD) have a well-defined pheno-endotype and a thoroughly characterized genetic background, which justifies its separate classification. The remaining non-CF bronchiectases (hereafter referred to simply as “bronchiectasis”) comprise a highly heterogeneous group of diseases, with marked clinical, prognostic, therapeutic, and particularly etiological differences [8]. From a functional perspective, bronchiectasis exhibits a wide range of patterns, including air trapping (70.2%), impaired diffusing capacity for carbon monoxide (DLCO) (55.7%), airflow obstruction (41.1%), hyperinflation (15.7%), and restrictive defects (8.0%). Notably, 9.7% of patients demonstrated normal pulmonary function [9].
Among the various dimensions of bronchiectasis, its etiology stands out as a principal source of heterogeneity across clinical presentations and geographic distributions [10]. While post-infectious forms (including post-tuberculous bronchiectasis) remain the most commonly recognized causes, those associated with COPD and asthma have shown a notable increase over time [11]. Idiopathic forms (defined as cases with no identifiable etiology, though not necessarily lacking one) are particularly variable across geographic regions [10]. It is conceivable that, with further advances in understanding, some etiological subtypes may be sufficiently characterized to merit separation from this current umbrella group in the near future, much like CF was in the past [12].
Currently, the frequency, distribution, and in some instances the temporal trends of bronchiectasis etiologies worldwide can be assessed due to the establishment of multiple registries in the early 21st century. These include both cross-sectional (n=3) and longitudinal (n=13) registries, encompassing national (n=15) and international (n=1) registries. The international registry (EMBARC) disaggregates data by country (n=27), thereby allowing for detailed analysis. The present manuscript provides a comprehensive review of the main etiological features of bronchiectasis, including prevalence, geographic distribution, and temporal evolution. This analysis is based both on data extracted from all currently available registries (some of them already published) and on selected other key publications that include relevant etiological information. Following a description of the methodology employed for data collection and general dataset characteristics, dedicated sections address each of the most frequent etiologies.
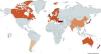
Data collectionBetween February 1st and June 30th, 2025, current etiological data were individually requested from the coordinators of the 16 national registries and one international registry currently in existence [10,13–25]. This included both active registries [10,13–17,19–23] and those that had already been closed for data collection [3,18,20,24]. Fig. 1 shows the general characteristics of the national e international registries of bronchiectasis in the world.
All registries contained etiological information, at least regarding the most frequent causes of bronchiectasis. These data were submitted to the coordinator of the present study by all registry coordinators except for three, who retained their data for imminent use in other specific studies [10,16,18]. For these three registries (with the permission of their coordinators), data were extracted from their most recent publications containing etiological information (one manuscript from 2024 and two from 2025).
An etiological table was ultimately completed (Table 1), detailing the year of data collection or direct obtaining of information by registry coordinators, the number of patients included, and the nine most common etiologies (including idiopathic), such as post-infectious, post-tuberculous, COPD- and asthma-related, allergic bronchopulmonary aspergillosis, primary ciliary dyskinesia, and immunodeficiencies. Less frequent etiologies were grouped under the category “Other.” In total, data were collected for 54,238 patients from 41 countries (since the international European registry [EMBARC] reports specific data of 27 european countries) [10]. The amount of missing data was remarkably low (only 8 items out of the 128 collected). Given that most registries are dynamic in nature, all coordinators were allowed to update their information during the manuscript review process until September 2025. In any case, the authors of the present manuscript believe that it should be considered more a review article than a systematic review, since in a previous short communication published in 2023 most of the etiologies of bronchiectasis by country were already presented according to their respective percentages [26]. The current manuscript provides an update to 2025 but does not include any statistical analysis of the available data
Most frequent etiologies in national and international registries of bronchiectasis.
| Registry | Year/datea | N° | ID | PI | P-TB | COPD | Asthma | ABPA | PCD | IDF | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARGENTINA | 2025 | 1196 | 17.4% | 26.3% | 16.8% | 9.9% | 9.7% | 1.5% | 1.8% | 2.6% | 14% |
| AUSTRALIA | 2025 | 854 | 30.8% | 39.3% | 1.9% | 5% | 6.8% | 4.1% | 4.2% | – | – |
| CANADA | 2025 | 289 | 33.6% | 15.9% | 4.5% | 14.2% | 11.6% | 4.4% | 7.6% | 5.2% | 3% |
| CHINA | 2024 | 10,324 | 29.6% | 43.2% | 12.2% | 4.9% | 1.5% | 1.3% | 0.6% | 0.3% | 6.4% |
| EUROPE | 2022 | 16,963 | 38.1% | 21.2% | 4.9% | 8.1% | 6.9% | 2.8% | 3% | 4.1% | 10.9% |
| GERMANY | 2025 | 2100 | 35.8% | 19.4% | 1.8% | 14.9% | 11.1% | 2.7% | 9% | 4.9% | 17.7% |
| INDIA | 2025 | 2195 | 21.4% | 22.4% | 35.5% | 5.3% | 2.5% | 8.9% | <1% | <1% | 2% |
| JAPAN | 2024 | 247 | 62.3% | 11.3% | 4.5% | 0.8% | – | 3.2% | 1.6% | 0.4% | – |
| KOREA | 2025 | 1140 | 36.7% | 21.8% | 21.3% | 4.2% | 4.7% | 0.5% | 0.4% | 0.5% | 9.9% |
| NEW ZEALAND | 2025 | 332 | 44.9% | 26.8% | 2.7% | 3% | 2.1% | 3.9% | 1.2% | 3% | 12.4% |
| SPAIN (OLD) | 2025 | 2099 | 24.2% | 30% | 18.6% | 7.8% | 5.4% | 0.9% | 2.9% | 9.4% | 0.8% |
| SPAIN (NEW) | 2025 | 2631 | 18.5% | 39.4% | 13.5% | 10.9% | 7.8% | 0.9% | 4.2% | 4.2% | 0.6% |
| TAIWAN | 2025 | 2753 | 24.2% | 13.9% | 14.7% | 26.7% | 10.1% | 0.4% | – | – | 9.9% |
| TURKISH | 2025 | 1035 | 45.4% | 39.5% | 11.3% | 0% | 0% | 0.1% | 1.5% | 1.3% | 0.9% |
| UK | 2025 | 1626 | 44% | 27% | 3% | 5% | 2% | 4% | 1% | – | 10% |
| USA | 2025 | 8454 | – | 3% | – | 18% | 26% | – | 3% | 4% | – |
| TOTAL | 54,238 | ||||||||||
| max | 62.3% | 43.2% | 35.5% | 26.7% | 26% | 8.9% | 9% | 9.4% | 14% | ||
| min | 17.4% | 3% | 1.8% | 0% | 0% | 0.1% | 0.4% | 0.3% | 0.6% | ||
| average | 33.8% | 25.1% | 12.3% | 9.1% | 7.9% | 2.3% | 3% | 3.2% | 6.7% |
Year/date: Data obtain from the last publication or the current information from the registry coordinators.
UK: United Kingdom; USA: United States; ID: Idiopathic; PI: Post-infectious; P-TB: Post-tuberculous; COPD: Chronic Obstructive Pulmonary Disease; ABPA: Allergic Bronchopulmonary Aspergillosis; PCD: Primary Ciliary Dyskinesia; IDF: Immunodeficiencies.
A universally accepted definition of “idiopathic bronchiectasis” is currently lacking. Rather than referring to cases without any identified etiology, it may be more accurate to describe idiopathic bronchiectasis as those cases in which the underlying cause remains unknown, either due to insufficient diagnostic evaluation or because the responsible condition has yet to be characterized (a less common scenario) [27].
Most national and international guidelines do not establish specific criteria for labeling bronchiectasis as idiopathic [28–30]. Instead, they recommend a minimum diagnostic workup to exclude treatable causes, supplemented by targeted testing based on clinical findings or suspicion of a specific etiology. The reported prevalence of idiopathic bronchiectasis varies widely across registries, ranging from 17.4% in Argentina [13] to 62.3% in Japan, with a global weighted mean of 33.8%.
This variability arises from several factors: 1. Sample size of each registry: Smaller registries are more susceptible to fluctuations in percentage values as new patients are added. This may be the case in Japan (62.3% idiopathic with only 247 individuals) or New Zealand (44.9% idiopathic with 332 patients); 2. Variability in diagnostic workup: The extent to which complementary tests are performed to confirm or rule out etiologies differs across countries, often due to disparities in resources, testing availability, or local clinical guidelines [31]. Two illustrative examples are: Gastroesophageal reflux disease (GERD), a potentially important contributor to bronchiectasis via microaspiration, often asymptomatic [32]. Nonetheless, diagnostic evaluation for GERD is rarely performed during etiological assessment, even in symptomatic patients, with treatment often defaulting directly to proton pump inhibitors. Another examples are immunodeficiencies, particularly subtle forms like IgG subclass deficiencies (quantitative or functional), which are frequently overlooked. A seminal study showed that systematic screening for these immunological parameters could reduce the proportion of idiopathic bronchiectasis by up to 17% [33]. These tests are particularly relevant, as many immunodeficiencies are potentially treatable [34], although a definitive causal relationship has not been established for most; 3. Relative prevalence of other etiologies: In regions with a past history of endemic tuberculosis or areas with limited access to healthcare, limited hygiene, and widespread poverty, post-infectious bronchiectasis is more prevalent thereby reducing the relative proportion of idiopathic cases. For instance, despite India's [18] limited medical infrastructure, idiopathic bronchiectasis accounts for only 21.4%, while post-infectious bronchiectasis (including post-tuberculous cases) accounts for 57.8%. Similarly, in Central/Eastern Europe [10], the idiopathic rate is 26.4%, counterbalanced by 40.8% of post-infectious cases; 4. Intracountry heterogeneity: In large nations such as China [16], the U.S. [25], Canada [15], or India [18], the prevalence of idiopathic bronchiectasis may differ substantially depending on the regions involved and their respective levels of participation. In China, most data originated from centers in the southeast, regions characterized by better medical infrastructure, while more impoverished areas were underrepresented. Within China itself, idiopathic bronchiectasis was less frequent in underprivileged regions (26.6%) than in more developed ones (30.8%), owing to a higher prevalence of post-tuberculous bronchiectasis in the former (16% vs. 10.6%) (16]. 5. Non-adherence to the established bronchiectasis guidelines regarding the recommended etiological investigations may increase the number of falsely classified idiopathic bronchiectasis cases [36].
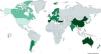
In summary, idiopathic bronchiectasis emerges as the most frequently reported etiology globally, with a worldwide average of 33.8%, surpassing even non-tuberculous post-infectious forms (Fig. 2).
Post-infectious bronchiectasisPost-infectious bronchiectasis—including cases related to non-tuberculous mycobacteria (NTM) are arguably the most challenging to interpret in terms of etiological attribution. These forms frequently entail substantial diagnostic uncertainty, as they are often labeled based on a history of viral or bacterial respiratory infections occurring many years prior (often in childhood), with the subsequent development of bronchiectasis being retrospectively attributed to those remote infections, despite the absence of preserved medical records or objective evidence [27].
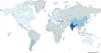
Only those cases with documented pneumonic processes for which historical imaging demonstrates bronchiectasis in the same anatomical location as the original infection can be considered reliably post-infectious. Nonetheless, it is reasonable to assume that post-infectious bronchiectasis is more prevalent in countries or regions with lower socioeconomic development and more limited healthcare infrastructure, as reflected in the global etiological registry data. For example, China [16] (43.2%) and Turkey [23] (39.5%) report rates significantly above the global mean (25.06%).
Interestingly, some high-income countries such as Spain [21] and Australia [14] also exhibit relatively high proportions of post-infectious bronchiectasis (39.4% and 39.3%, respectively). This may reflect a greater tendency to associate adult bronchiectasis with early-life respiratory infections, although other circumstances could account for this phenomenon (Fig. 3).
A particularly complex relationship exists between bronchiectasis and non-tuberculous mycobacterial (NTM) infections. The directionality of this association remains unclear—NTMs may act as causative agents of bronchiectasis, or alternatively, they may establish chronic airway infection in individuals with pre-existing structural lung damage [35,36]. Cross-registry comparisons are further complicated by differences in registry design and enrollment criteria: most registries exclude bronchiectasis with active NTM infection; the U.S. registry explicitly recruits patients with NTM infection (with or without bronchiectasis); and the Japanese registry enrolls both bronchiectasis and NTM pulmonary disease (NTM-PD) [37]. Separately, the nodular–bronchiectatic (NB) form of NTM-PD—predominantly affecting postmenopausal women without prior lung disease—has been increasingly recognized [38]. These design choices shape the observed etiologic proportions. For example, in the U.S. registry, post-infectious bronchiectasis accounts for fewer than 3% of cases, likely reflecting NTM-focused inclusion criteria [25].
Post-tuberculous bronchiectasisA situation somewhat analogous to that of post-infectious bronchiectasis arises in cases of post-tuberculous bronchiectasis, although the level of diagnostic certainty may be higher for two main reasons. First, in certain countries or regions with a history of endemic tuberculosis, a significant burden of post-tuberculous bronchiectasis has emerged among patients previously affected. Second, upper lobe-predominant bronchiectasis in individuals with a self-reported history of tuberculosis, particularly when supported by prior radiological documentation, strongly suggests a causal relationship [39].
Furthermore, the association between tuberculosis and poor socioeconomic conditions is well established. Accordingly, countries such as India [18] (35.5%), South Korea [19] (21.3%), historical Spanish cohort [20] (18.6%), and regions in Eastern Europe [10] or underprivileged areas of China [16] report the highest prevalence of post-tuberculous bronchiectasis. In contrast, the prevalence in Western Europe [10], Australia/New Zealand [14], Canada [15], the UK [24], and Japan remains below 5% (Fig. 4).
COPD-associated bronchiectasisRecent years have witnessed growing interest in the relationship between bronchiectasis and chronic obstructive pulmonary disease (COPD) [1], as their coexistence is associated with worse clinical outcomes, including increased mortality—particularly in COPD—and greater disease severity, often due to a higher rate of exacerbations. It is estimated that 35–50% of patients with severe COPD and up to 25% of those with severe asthma may have clinically significant bronchiectasis [40,41]. However, the label of COPD is offen applied with bronchiectasis patients who do not have an objective evidence of airflow obstruction or a smoking history [1].
When bronchiectasis is the primary diagnosis, the proportion of patients with coexisting COPD varies widely by country—from 0.8% in Japan to 26.7% in the U.S. [25], with a global average of 9.1%. Remarkably high percentages in Taiwan [22] (26.7%) and the U.S. [25] (18%) may reflect inclusion of patients for whom COPD is the primary diagnosis and bronchiectasis a secondary development. Polverino et al., using data from the EMBARC registry and applying the ROSE (Radiology, Obstruction, Symptoms, Exposure) criteria [42] found evidence of COPD overdiagnosis. Specifically, 22.2% of patients labeled with COPD in the registry did not exhibit airflow obstruction, and 31.9% had no history of smoking exceeding 10 pack-years. Consequently, the estimated prevalence of COPD among patients with bronchiectasis was 13% [1].
Nonetheless, establishing a definitive causal relationship between COPD and bronchiectasis is difficult, given the high prevalence of both diseases and shared risk factors. The biological plausibility of bronchiectasis developing in COPD patients is supported by evidence of intense neutrophilic inflammation and a high prevalence of chronic bronchial infection [43]. A longitudinal study showed that up to 25% of COPD patients without baseline bronchiectasis developed it over 10 years of follow-up, in the absence of other identifiable causes [44].
In Spain, a comparison between historical and contemporary registries spanning 15 to 20 years reveals a notable increase in the proportion of bronchiectasis cases attributed to or associated with COPD, possibly due to increased clinical attention to this association [20,21].
Asthma-associated bronchiectasisThe prevalence of asthma among bronchiectasis patients, according to global registry data, tends to remain below 10–12% in most countries. The exceptionally high rate observed in the U.S. registry (26%) likely reflects asthma being recorded as a comorbidity rather than a true etiological factor [25]. Conversely, countries like Turkey report a prevalence of 0% for both asthma and COPD as underlying causes of bronchiectasis [23], which may suggest that these conditions are not regarded as recognized etiologies—potentially contributing to Turkey's leading percentage of post-infectious bronchiectasis and second-highest rate of post-tuberculous forms (after China).
Unlike COPD, the pathophysiological correlation between asthma and bronchiectasis remains elusive. Most forms of asthma—excluding neutrophilic variants—are characterized by eosinophilic inflammation, which, although proinflammatory, is not generally considered sufficient to damage bronchial walls in the same way as neutrophilic processes [45]. Furthermore, bacterial chronic infection is far less frequent in asthma patients, possibly due to less routine microbiological evaluation.
To date, no longitudinal studies have established a causal link between asthma and bronchiectasis. Diagnostic confusion may arise due to overlapping symptoms: some bronchiectasis patients may be misdiagnosed with asthma in the absence of chest CT imaging, while others may have undiagnosed asthma due to limited availability of advanced tests such as exhaled nitric oxide or bronchoprovocation testing [46]. This may help explain the low reported prevalence in countries like China [16] (1.5%), New Zealand (2.1%) and India [18] (2.5%). The global average stands at 7.9%.
As with COPD, a comparison of historical and recent Spanish registries reveals a significant increase in asthma-related bronchiectasis over time [20,21]. Finally, the recent identification of eosinophilic bronchiectasis with a Th2-high profile (defined by >300eosinophils/μL in peripheral blood) warrants attention [46,47]. Although such cases exhibit distinct clinical features, it remains uncertain whether they represent true asthma phenotypes with comorbid bronchiectasis or a distinct endotype of eosinophilic bronchiectasis. Finally, patients with coexisting bronchiectasis and asthma exhibited an increased risk of exacerbations but demonstrated improved outcomes with inhaled corticosteroid (ICS) therapy. Unexpectedly, patients with both bronchiectasis and asthma had significantly lower mortality rates compared with those without asthma [2].
Allergic bronchopulmonary aspergillosis associated bronchiectasisA strong bidirectional relationship exists between ABPA and bronchiectasis. While the prevalence of bronchiectasis in ABPA exceeds 70–80% over the course of the disease, it often presents with characteristic features such as central distribution, upper lobe involvement, high-attenuation mucus plugging, and is even included as a minor diagnostic criterion for ABPA itself. Bronchiectasis contributes to the frequency of pulmonary infections, thereby worsening the progression, prognosis, and treatment response of ABPA [48].
Despite this close association, ABPA as an etiological cause of bronchiectasis is relatively uncommon in the global registry data presented in this study, accounting for no more than 4–5% of cases, with a global mean of 2.3%. This low figure may partially reflect underdiagnosis, particularly when appropriate diagnostic testing is not performed, and ABPA is instead misclassified as difficult-to-control asthma (a major diagnostic criterion for ABPA), thereby artificially reducing its attribution as a bronchiectasis etiology [48].
Primary ciliary dyskinesia associated bronchiectasisPrimary ciliary dyskinesia (PCD) is a rare, genetically heterogeneous, multisystem disorder with autosomal recessive, X-linked, or autosomal dominant inheritance, caused by dysfunction of motile cilia. Over 50 pathogenic variants have been identified, resulting in marked clinical variability. PCD may present as Kartagener syndrome—comprising situs inversus, bronchiectasis, and chronic sinonasal disease [49].
Although congenital in origin, pulmonary symptoms may not manifest until adulthood and typically include recurrent infections of the upper and lower respiratory tract. The prevalence of bronchiectasis in adults with PCD is extremely high (80–100%), as mucociliary clearance is impaired from early life, predisposing to chronic bacterial colonization [50].
One of the most significant studies is that of Ewen et al., based on 1000 patients from the German bronchiectasis registry (PROGNOSIS), where PCD was the fifth most common cause and characterized by a distinct phenotype: younger age, chronic rhinosinusitis and/or nasal polyps, radiological involvement of middle and lower lobes, longer disease duration, and frequent isolation of Pseudomonas aeruginosa[51].
Although very low nasal nitric oxide (NO) levels have been proposed as a diagnostic clue in PCD-related bronchiectasis, recent studies show that certain PCD-related mutations may exhibit normal nasal NO despite the presence of bronchiectasis, thereby limiting its diagnostic utility [52].
The reported prevalence of PCD as an underlying cause of bronchiectasis is highly dependent on diagnostic capacity and clinical suspicion. In the German PROGNOSIS registry [17], PCD accounts for 9% of cases, while other registries rarely exceed 4%. In low-and middle-income countries (LMIC) such as India [18] or parts of China [16], diagnosis rates remain below 1%. However, even in resource-rich countries like the UK [24], PCD is identified in only 1% of bronchiectasis cases, likely due to limited overlap between (predominantly pediatric institutions caring for people with PCD and bronchiectasis, respectively).
Although curative therapy is not currently available, identification of PCD is critical for genetic counseling and fertility management. Given the advances in molecular genetics, some authors argue that PCD may eventually be considered a distinct bronchiectasis endotype, akin to the classification of bronchiectasis due to (CF).
Immunodeficiency-associated bronchiectasisA broad spectrum of immunodeficiencies including those primary and secondary, humoral, cellular, or combined have been associated with bronchiectasis. These are typically grouped under “immunodeficiency-associated bronchiectasis” in registries, the majority being humoral in nature [53]. Globally, such cases account for 3.2% of all etiologies, although this may vary based on cohort composition. For instance, in the historical Spanish registry [20], immunodeficiencies represented 9.4% due to a high number of patients enrolled from a specialized immunodeficiency unit.
Secondary immunodeficiencies such as those due to chronic renal failure, hematologic malignancies, amyloidosis, immunosuppressive drugs (e.g., corticosteroids, chemotherapy, HIV infection, or post-lung transplantation—typically account for less than 1% and are often considered separately [6,7,20].
Aliberti et al. demonstrated in a cohort of 400 patients that the detection rate of immunodeficiency is strongly dependent on the extent of testing. A basic screen (CBC and total immunoglobulin levels) may identify immunodeficiency in 8.9% of cases, while extended testing, such as IgG subclass quantification and lymphocyte subset analysis, can raise detection rates to over 20% and 44.6%, respectively [53].
Most immunodeficiencies identified are primary humoral disorders and are believed to contribute to bronchiectasis through recurrent infections, particularly with encapsulated organisms. However, such studies reveal associations rather than causation, and a comprehensive etiologic workup should be pursued to identify more plausible causes [34].
Among primary immunodeficiencies, Common Variable Immunodeficiency (CVID) is most strongly associated with bronchiectasis, due to its hallmark features of IgG deficiency, frequent IgA/IgM deficits, and poor vaccine response. The prevalence of bronchiectasis in CVID ranges from 34 to 52%, influenced by age, diagnostic delay, and severity [54]. Other relevant conditions include selective IgA deficiency (5–15%) and isolated or combined IgG subclass deficiencies. X-linked agammaglobulinemia, characterized by near-total absence of immunoglobulins, has bronchiectasis rates of 30–70% but is typically diagnosed in early childhood [55].
A current topic of uncertainly is the relationship between IgG subclass deficiencies, especially IgG2 (often with IgG4 or IgA) and bronchiectasis, due to their association with recurrent respiratory infections by encapsulated pathogens. Rodrigo et al. reported that some patients may exhibit functional immunodeficiencies (i.e., poor vaccine response) despite normal or elevated serum IgG/subclass levels [56]. In older adults (≥65 years), such latent deficiencies may contribute to bronchiectasis, although this remains speculative [57]. Although low immunoglobulin levels and functional antibody testing can identify many immunodeficiencies, referral to an immunologist should be considered for patients with suggestive features, even when initial immunoglobulin levels are normal [59].
Current guidelines generally recommend IgG subclass testing only when other causes have been excluded and there is a compatible history of recurrent upper or lower respiratory infections since childhood [28–30]. Identification is important, however, as many primary humoral immunodeficiencies are amenable to replacement therapy [55]. Many other immune disorders, whether cellular, humoral, or mixed, are described but are exceedingly rare as primary causes.
Other causesAcross the global bronchiectasis etiology table, less common causes (categorized as “Other”) rarely exceed 2–3% and in most registries account for <1% of cases. These include conditions such as: Rheumatoid arthritis, Gastroesophageal reflux disease (GERD), inflammatory bowel disease, vasculitis, diffuse panbronchiolitis, local obstructive causes, alpha-1 antitrypsin deficiency, connective tissue disorders, congenital malformations, yellow nail syndrome, Pink disease, Young syndrome, chronic rhinosinusitis, hematologic and lymphoproliferative malignancies [6,7,20]. Notably, traction bronchiectasis in patients with, interstitial lung disease, or secondary to SARS-CoV-2 infection are excluded from the classification.
Despite their rarity, these causes should be actively considered, particularly because some—such as alpha-1 antitrypsin deficiency and GERD have specific treatments. Moreover, controlling the underlying disease, especially in the case of rheumatoid arthritis, inflammatory bowel disease, and Sjögren syndrome, for which effective biologics now exist, can help stabilize bronchiectasis progression [58]. For example, rheumatoid arthritis in associated with increase mortality in bronchiectasis (de Soyza) [60] and new biological treatments could stabilize the progression of inflammatory bowel disease [61].
National or international bronchiectasis registries are still absent across extensive regions including the Russian Federation and its republics, most African and Asian countries, and much of Latin America (apart from Argentina). In these settings, post-infectious and post-tuberculous bronchiectasis almost certainly predominate, mirroring the high burden of communicable diseases in socio-economically disadvantaged populations [62–65].
For instance, a study from Nigeria reported that nearly 60% of bronchiectasis cases were post-tuberculous. Similarly, in small series from Brazil and Chile (with considerable regional socioeconomic variation), idiopathic and post-infectious forms remain the most frequently reported etiologies.
ConclusionThe present review provides a comprehensive overview of the main etiologies of bronchiectasis worldwide, based on data extracted from major national and international registries. Idiopathic bronchiectasis emerges as the most frequent subtype, followed by post-infectious and post-tuberculous forms. Notably, the striking geographical variability underscores the influence of diverse social, logistical, and healthcare-related factors. A stronger adherence to both national and international bronchiectasis guidelines is undoubtedly required to reduce the proportion of cases classified as idiopathic—or more accurately, of unknown etiology—since several underlying causes of bronchiectasis are potentially treatable, thereby improving patient prognosis [66].
ContributionsStudy design: MAMG.
Data acquisition: TA, TAs, LB, CCL, RMG, WJG, DK, YMO, MP, FCR, TS, CT, and CW.
Data interpretation and writing the manuscript: MAMG.
All authors critically reviewed the manuscript, and approved its final, submitted, version.
Use of artificial intelligenceNo. The translation into English was made by a native speaker with more than 20 years of experience.
Financial supportNo.
Conflict(s) of interestNo.