Bronchiectasis is a chronic respiratory disease characterized by abnormal airway dilation, excessive mucus production, and recurrent respiratory infections [1]. Although the pathogenesis of bronchiectasis is not fully understood, an abnormal pulmonary immunity and chronic bronchial infection, particularly with Pseudomonas aeruginosa, are central in its pathophysiology and natural history [2,3]. Both the pulmonary immunity and the bronchial infection are associated to worse quality of life, exacerbations and mortality [4–7]. In addition, other studies have suggested that alterations in patterns of systemic inflammation have also been related to worse clinical outcomes [8,9]. However, available data on the peripheral immunity and their relationship with chronic infection remains limited. Here, we hypothesize that patients with bronchiectasis, and especially those patients with chronic airway infection by P. aeruginosa, have an altered peripheral blood immune profile. Therefore, this study aimed to characterize the systemic innate and adaptive immune cells of patients with bronchiectasis and to examine their relationship with chronic bronchial infection by P. aeruginosa.
This is an observational study with two components: (1) a cross-sectional analysis comparing sociodemographic, clinical and peripheral immune profile data, between patients with bronchiectasis vs. healthy controls, and between those bronchiectasis patients with vs. without chronic P. aeruginosa; and (2) a longitudinal analysis of a subset of patients with bronchiectasis. We included patients with clinically stable bronchiectasis who attended the Respiratory Department at Hospital Clinic (Barcelona, Spain) and Bellvitge Hospital (Hospitalet de Llobregat, Spain) between 2021 and 2023, and healthy controls, who had normal lung function, no active respiratory, cancer or autoimmune disease, nor immunosuppressive treatment. The study was approved by the local Ethics Committee (HCB/2021/0310) and all participants signed their informed consent. Bronchiectasis diagnosis and aetiology were determined following current guidelines [10,11]. Exclusion criteria were primary immunodeficiency, terminal illness, active malignant disease, human immunodeficiency virus (HIV) or prolonged use of corticosteroids (>20mg per day for at least 4 weeks). Disease severity were determined using the Bronchiectasis Severity Index (BSI) [12] and the FACED score [13], and chronic bronchial infection by P. aeruginosa was defined as two or more isolates at least three months apart in one year [11]. Clinical characteristics, lung function, microbiological data and peripheral blood samples were collected in a single visit, 7±6 years after diagnosis. Fluorescence-activated cell sorting (FACS) was used to profile B and T cells (and subpopulations), natural killer (NK) cells, NKT-like cells, monocytes, neutrophils and eosinophils, as previously described [14]. Patients were stratified according to the presence of chronic P. aeruginosa infection. In a subset of patients followed for 12 months after inclusion, peripheral blood was collected again for immune phenotyping reassessment. Comparisons between the study groups were performed using t-test or Mann–Whitney test, as appropriate, and all analyses were adjusted for age sex.
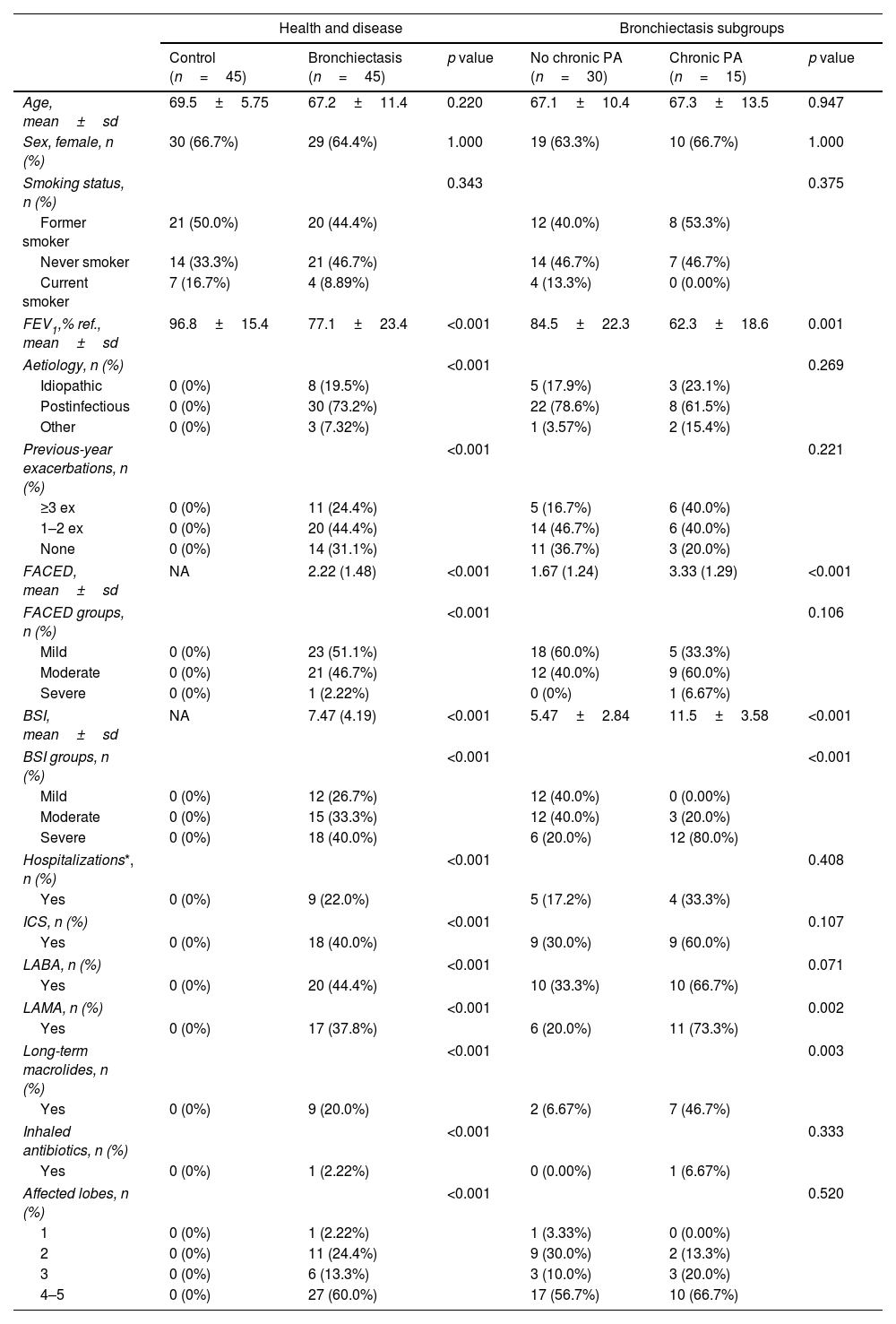
A total of 45 adults with stable bronchiectasis and 45 age-sex-matched healthy controls, were included in the study. Table 1 summarizes the demographic and clinic characteristics of the participants. Chronic P. aeruginosa infection was present in 33% of patients. Compared to those without infection, these patients had more severe disease, as reflected by higher BSI and FACED scores, lower FEV1, more frequent exacerbations, and greater use of chronic respiratory treatments.
Clinical characteristics of patients with stable bronchiectasis and age-sex-matched controls.
| Health and disease | Bronchiectasis subgroups | |||||
|---|---|---|---|---|---|---|
| Control (n=45) | Bronchiectasis (n=45) | p value | No chronic PA (n=30) | Chronic PA (n=15) | p value | |
| Age, mean±sd | 69.5±5.75 | 67.2±11.4 | 0.220 | 67.1±10.4 | 67.3±13.5 | 0.947 |
| Sex, female, n (%) | 30 (66.7%) | 29 (64.4%) | 1.000 | 19 (63.3%) | 10 (66.7%) | 1.000 |
| Smoking status, n (%) | 0.343 | 0.375 | ||||
| Former smoker | 21 (50.0%) | 20 (44.4%) | 12 (40.0%) | 8 (53.3%) | ||
| Never smoker | 14 (33.3%) | 21 (46.7%) | 14 (46.7%) | 7 (46.7%) | ||
| Current smoker | 7 (16.7%) | 4 (8.89%) | 4 (13.3%) | 0 (0.00%) | ||
| FEV1,% ref., mean±sd | 96.8±15.4 | 77.1±23.4 | <0.001 | 84.5±22.3 | 62.3±18.6 | 0.001 |
| Aetiology, n (%) | <0.001 | 0.269 | ||||
| Idiopathic | 0 (0%) | 8 (19.5%) | 5 (17.9%) | 3 (23.1%) | ||
| Postinfectious | 0 (0%) | 30 (73.2%) | 22 (78.6%) | 8 (61.5%) | ||
| Other | 0 (0%) | 3 (7.32%) | 1 (3.57%) | 2 (15.4%) | ||
| Previous-year exacerbations, n (%) | <0.001 | 0.221 | ||||
| ≥3 ex | 0 (0%) | 11 (24.4%) | 5 (16.7%) | 6 (40.0%) | ||
| 1–2 ex | 0 (0%) | 20 (44.4%) | 14 (46.7%) | 6 (40.0%) | ||
| None | 0 (0%) | 14 (31.1%) | 11 (36.7%) | 3 (20.0%) | ||
| FACED, mean±sd | NA | 2.22 (1.48) | <0.001 | 1.67 (1.24) | 3.33 (1.29) | <0.001 |
| FACED groups, n (%) | <0.001 | 0.106 | ||||
| Mild | 0 (0%) | 23 (51.1%) | 18 (60.0%) | 5 (33.3%) | ||
| Moderate | 0 (0%) | 21 (46.7%) | 12 (40.0%) | 9 (60.0%) | ||
| Severe | 0 (0%) | 1 (2.22%) | 0 (0%) | 1 (6.67%) | ||
| BSI, mean±sd | NA | 7.47 (4.19) | <0.001 | 5.47±2.84 | 11.5±3.58 | <0.001 |
| BSI groups, n (%) | <0.001 | <0.001 | ||||
| Mild | 0 (0%) | 12 (26.7%) | 12 (40.0%) | 0 (0.00%) | ||
| Moderate | 0 (0%) | 15 (33.3%) | 12 (40.0%) | 3 (20.0%) | ||
| Severe | 0 (0%) | 18 (40.0%) | 6 (20.0%) | 12 (80.0%) | ||
| Hospitalizations*, n (%) | <0.001 | 0.408 | ||||
| Yes | 0 (0%) | 9 (22.0%) | 5 (17.2%) | 4 (33.3%) | ||
| ICS, n (%) | <0.001 | 0.107 | ||||
| Yes | 0 (0%) | 18 (40.0%) | 9 (30.0%) | 9 (60.0%) | ||
| LABA, n (%) | <0.001 | 0.071 | ||||
| Yes | 0 (0%) | 20 (44.4%) | 10 (33.3%) | 10 (66.7%) | ||
| LAMA, n (%) | <0.001 | 0.002 | ||||
| Yes | 0 (0%) | 17 (37.8%) | 6 (20.0%) | 11 (73.3%) | ||
| Long-term macrolides, n (%) | <0.001 | 0.003 | ||||
| Yes | 0 (0%) | 9 (20.0%) | 2 (6.67%) | 7 (46.7%) | ||
| Inhaled antibiotics, n (%) | <0.001 | 0.333 | ||||
| Yes | 0 (0%) | 1 (2.22%) | 0 (0.00%) | 1 (6.67%) | ||
| Affected lobes, n (%) | <0.001 | 0.520 | ||||
| 1 | 0 (0%) | 1 (2.22%) | 1 (3.33%) | 0 (0.00%) | ||
| 2 | 0 (0%) | 11 (24.4%) | 9 (30.0%) | 2 (13.3%) | ||
| 3 | 0 (0%) | 6 (13.3%) | 3 (10.0%) | 3 (20.0%) | ||
| 4–5 | 0 (0%) | 27 (60.0%) | 17 (56.7%) | 10 (66.7%) | ||
FEV1: forced expiratory volume in 1second; FACED: acronym for FEV1, age, chronic colonization, extension and dyspnoea; BSI: Bronchiectasis Severity Index; ICS: inhaled corticosteroids; LABA: long-acting beta-agonists; LAMA: long-acting muscarinic antagonists; Hospitalizations*: number of hospitalizations due to an exacerbation episode.
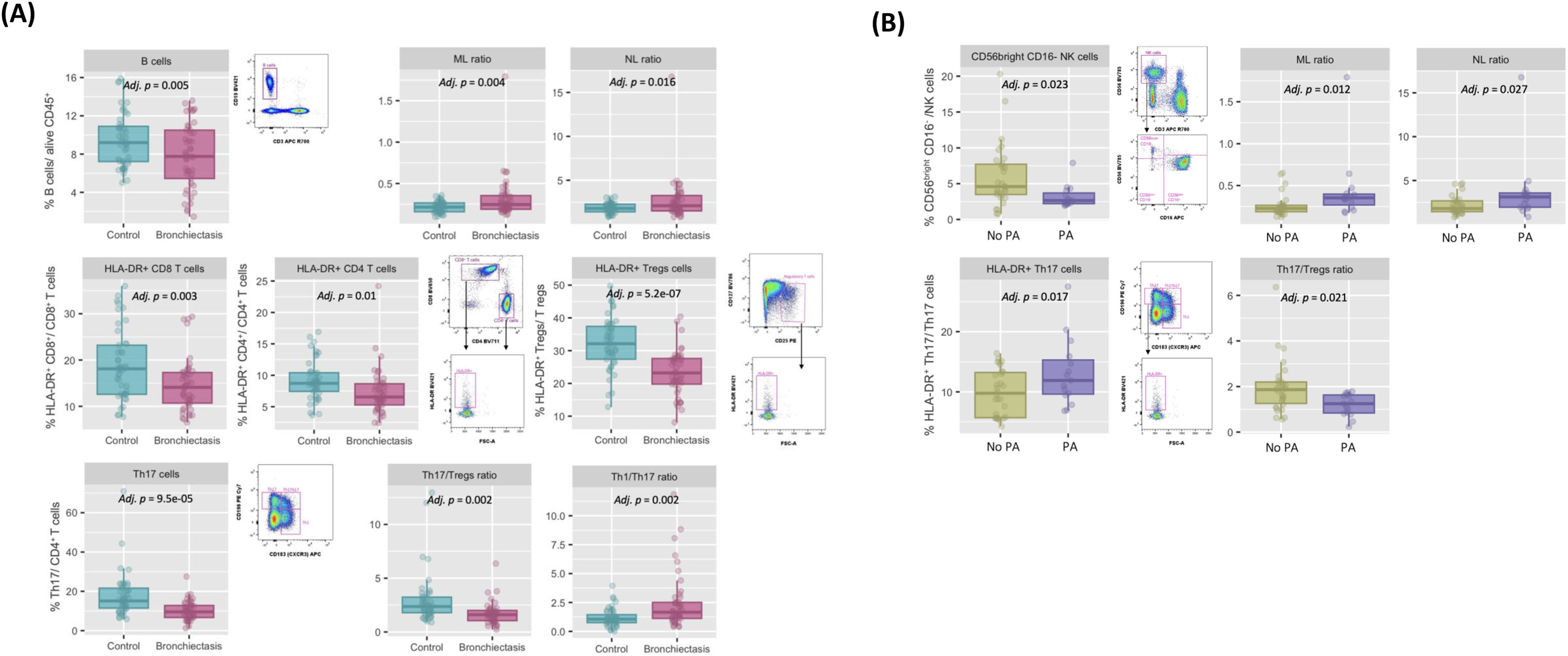
In the immune profiling, compared to controls, patients with bronchiectasis presented lower percentages of circulating lymphocytes (adjusted (adj.) p=0.015) and B cells (adj. p=0.005), and the cellular ratios, monocyte-to-lymphocyte ratio (MLR) (adj. p=0.004) and neutrophil-to-lymphocyte ratio (NLR) (adj. p=0.016) were also altered (Fig. 1A). Additionally, the proportions of HLA-DR+CD8+ (i.e. cytotoxic activated T cells), HLA-DR+CD4+ (i.e. activated helper T cells) were also lower (adj. p=0.003 and adj. p=0.01, respectively) in patients with bronchiectasis. Interestingly, within the T CD4+ population, the proportion of HLA-DR+ (i.e. activated) T regulatory (T regs) cells, defined as CD25+CD127−CD4+CD3+ T cells, was also lower in bronchiectasis, compared to healthy controls (adj. p=5.2e−07). Th17 lymphocytes, defined as CD183−CD196+CD4+CD3+ T cells, were decreased in bronchiectasis (adj. p=9.5e−05) and, accordingly, the peripheral Th1/Th17 and Th17/Tregs ratios were also altered (adj. p=0.002 and adj. p=0.002, respectively) (Fig. 1A). Compared to those without infection, patients with chronic P. aeruginosa presented higher levels of neutrophils (adj. p=0.021), monocytes CD14+ (adj. p=0.042), increased MLR (adj. p=0.012) and NLR (adj. p=0.027), along with a decrease in the proportion of the CD56brightCD16+ NK cell subpopulation (adj. p=0.023). Regarding the T lymphocyte subsets, although patients with chronic P. aeruginosa infection had an increase in the percentage of HLA-DR+ (i.e. activated) Th17 (adj. p=0.017), they presented an inverted (decreased) Th17/Tregs ratio (adj. p=0.021) (Fig. 1B).
Characterization of the peripheral innate and adaptive immune compartment in n=45 clinically stable bronchiectasis patients: (A) compared to n=45 age-sex-matched healthy controls, adjusted by age and sex: Controls (blue box) vs. Bronchiectasis (pink box) (median [IQR]); (B) categorized by the presence of chronic airway P. aeruginosa infection (PA), adjusted by age and sex: No PA (green box) vs. PA (lila box) (median [IQR]).
In a subset of 24 patients with bronchiectasis, blood immunophenotyping was repeated at 12 months and compared to baseline values, stratified by the presence of chronic P. aeruginosa infection. Interestingly, within each group, key immune parameters and populations, including NLR, MLR, CD56brightCD16+ NK cell subpopulation and HLA-DR+ Th17, remained stable over time. The only significant longitudinal change observed was a reduction in the Th17/Treg ratio in the group with chronic P. aeruginosa (p=0.031).
Our analysis shows that bronchiectasis is associated with alterations in peripheral immune cells, especially in those patients with chronic airway infection by P. aeruginosa. Systemic inflammatory mediators and their association with disease severity have recently gained attention in bronchiectasis. In line with data reported by Martinez-García et al. [15], our results not only showed notable differences in the NLR and MLR in patients with P. aeruginosa infection, but also that these differences are disease specific when compared to age-sex-matched controls. In bronchiectasis, not only innate, but also adaptive immune cells are altered. Microbial exposure can have a direct effect on cellular traffic, and on the modulation of lymphopoiesis leading to lymphopenia [16]. Thus, reduced blood proportions of activated T cells and B cells have previously been related to persistent immune activation, inflammation, apoptosis and immune “exhaustion”, as seen in chronic infections and other respiratory diseases [14]. In COPD, frequent exacerbators showed an imbalance favouring pro-inflammatory Th17 cells over regulatory T cells, which has been implicated in the pathogenesis of the disease [17]. Similarly, impaired Tregs functions, accompanied by an increase Th17 response, associated to neutrophilic inflammation and worse clinical outcomes, has also been observed in asthma. These findings highlight the importance of T1h7/Tregs ratio against lung infections [18]. Here, although an altered balance was observed in patients with bronchiectasis, especially those with chronic P. aeruginosa infection, the pattern differed from that described in COPD and asthma, showing lower levels of Th17, but with an activated phenotype.
These findings may suggest two possible scenarios. The first one being that a reduction in Th17 cells and the corresponding relative increase in Tregs can represent a shift in the immune environment towards a more tolerant state. This altered balance, together the reduction of peripheral activated T, B cells and the immunoregulatory CD56brightCD16+ NK cell subpopulation may inhibit inflammatory responses and foster an incomplete resolution of the infection, leading to a susceptible environment for chronic infections. This tolerant profile can be also seen as a protective mechanism to avoid an excessive immune response and further lung damage. Supporting this, a decrease in activated lymphocytes, was described in patients with sepsis with fatal outcomes [19]. Alternatively, another plausible explanation might not consider the reduction of these peripheral immune cells as a sign of immune depletion, but a consequence of enhanced migration to the inflamed tissue. This hypothesis is supported by lung biopsy studies in bronchiectasis which reported increased airway inflammation characterized by CD45+ infiltrates, particularly CD4+ T cells, macrophages and neutrophils [20]. To completely understand these immune mechanisms involved, both lung tissue and blood from the same individuals should be analyzed. Finally, the baseline immune profile associated with chronic bronchial infection remained stable after 12 months. This consistency has been shown in previous IPF studies, where the immunophenotype associated with a progressive phenotype at diagnosis, persisted over time [14], and suggests a disease specific effect on blood counts.
To our knowledge, a key strength of this study is the broad profiling of blood immune cells, an accessible tissue in clinical practice and research, along with the 12-month follow-up to assess changes over time. The main limitation is the relatively small, which restricts the ability to prospectively link the peripheral immunophenotypes with clinical outcomes (i.e. risk of future exacerbations). In addition, the effect of treatments such as long-term macrolides use and/or inhaled antibiotic therapies on the peripheral immunophenotype should be further explored. Moreover, future studies should focus on the interplay between host systemic immune populations and a broader microbiome context in bronchiectasis, in order to better understand their interactions with different pathogens [21].
In conclusion, we demonstrated that patients with bronchiectasis have an altered systemic immune profile towards a less active or tolerant phenotype, especially those with chronic bronchial infection by P. aeruginosa, and that it persists over time. Altogether, these findings offer new insights into the systemic component of bronchiectasis and chronic infection, providing potential biomarkers linked to disease heterogeneity and severity.
Contribution of each authorStudy conception and design: RF, OS.
Data acquisition: BS, RA, GSC, AP, PM, MV, AV, FM, NS, OS.
Data analysis: BS, NM, LP, RF, OS.
Manuscript preparation: BS, NM, LP, RF, OS.
Manuscript revision and approval: All.
Artificial intelligenceNo artificial intelligence involvement.
Funding of the researchThis work was supported by Contractes Clínic de Recerca “Emili Letang – Josep Font” 2021 (Belen Solarat), Instituto de Salud Carlos III (PI21/00419, CD23/00093), SEPAR, SOCAP, FUCAP.
Conflicts of interestStefano Aliberti: Grants/Contracts: GSK.
Amelia Shoemark: Grants/Contracts: AstraZeneca, Lifearc.
James D. Chalmers: Grants/Contracts: Astrazeneca, Genetech, Insmed, Novartis, Gilead Sciences, Glaxosmithkline, Grifols, Boehringer Ingelheim.
Rosa Faner: Grants/contracts: Menarini, GSK, AstraZeneca, Sanofi, Chiesi, ERC-CoG-PredictCOPD-GA101044387, ISC-III, Agaur, ICREA, H2020, SEPAR, ERS, and Serra Húnter.
Oriol Sibila: Consulting fees: Insmed and Boehringer Ingelheim.
The remaining authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
The authors thank all participants in the study for their willingness to contribute to medical research, all field workers for their dedication and quality of their daily work, and the Flow Cytometry and Cell Sorting core facility of the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS).


![Characterization of the peripheral innate and adaptive immune compartment in n=45 clinically stable bronchiectasis patients: (A) compared to n=45 age-sex-matched healthy controls, adjusted by age and sex: Controls (blue box) vs. Bronchiectasis (pink box) (median [IQR]); (B) categorized by the presence of chronic airway P. aeruginosa infection (PA), adjusted by age and sex: No PA (green box) vs. PA (lila box) (median [IQR]). Characterization of the peripheral innate and adaptive immune compartment in n=45 clinically stable bronchiectasis patients: (A) compared to n=45 age-sex-matched healthy controls, adjusted by age and sex: Controls (blue box) vs. Bronchiectasis (pink box) (median [IQR]); (B) categorized by the presence of chronic airway P. aeruginosa infection (PA), adjusted by age and sex: No PA (green box) vs. PA (lila box) (median [IQR]).](https://static.elsevier.es/multimedia/03002896/unassign/S0300289625003825/v1_202511050425/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)







