Peripheral eosinophils can increase in case of allergic, parasitic, or auto-immune disorders. Absolute and relative counts of peripheral eosinophils have been proposed to prescribe drugs in asthmatic patients and to assess their effectiveness.1 Their assessment was also suggested in individuals with chronic obstructive pulmonary disease (COPD). Eosinophils counts >300cells/μL or >3% have been associated with a higher incidence and severity of exacerbations but with a better response to inhaled corticosteroids (ICs).2 On the other hand, counts <100eosinophils/μL (<2%) has been associated with poor therapeutic response to ICs and a high incidence of adverse events (e.g., pneumonia).3
There is very scarce literature on the role played by peripheral eosinophils in patients with bronchiectasis. It is known that bronchiectasis patients have a higher eosinophil counts in bronchial mucosa in comparison with healthy subjects and, sometimes, an eosinophilic pattern can be the predominant inflammatory cell type in the sputum.4 Keeping into account the bactericidal activity of eosinophils,5 the prognostic value of bronchial bacterial infection and its association with eosinophil counts,6 and the potential immunosuppressive effect of ICs, an interaction between peripheral eosinophils and ICs treatment could be hypothesized also in bronchiectasis. Only one small study conducted by Aliberti et al. found that bronchiectasis patients treated with ICs can show a significant improvement of quality of life only when eosinophilia (at least 150cells/L or ≥3%) is found.7
Usually, baseline counts of circulating eosinophils in patients with clinical stability is used to predict long-term prognosis and therapeutic response, following the demonstration of a correlation between lung tissue and circulating eosinophil counts.8 However, peripheral eosinophils can fluctuate overtime because of their short half-life and diurnal variation (e.g., peak in the evening).9 Therefore, before their assessment as a biomarker in large studies in bronchiectasis, it is key to evaluate their time stability, as well as the role played by ICs at different dosages. A randomized controlled trial (RCT) published in 2008 evaluated the effectiveness of ICs in patients with bronchiectasis and performed repeated measures of eosinophils at different time-points.10
The objective of the present study was to assess the variability of circulating eosinophils overtime retrieving data from that RCT, as well as to prove ICs effect on this variability when prescribed at different dosages.
The RCT recruited 132 patients with bronchiectasis diagnosed through high-resolution CT scan. Patients with cystic fibrosis, asthma, allergic bronchopulmonary aspergillosis, those exposed to systemic steroids or immunosuppressive therapy, or suffering from eosinophilic pulmonary or systemic diseases were excluded. Finally, 86 were randomized and followed-up for the entire duration of the study. All randomized patients were not exposed to ICs in the three months before their enrollment. A total of 28 patients did not receive ICs, 29 were treated with 250μg of fluticasone propionate (FP) bid (medium doses), and 29 were exposed to 500 ug of FP bid (high doses) for 6 months. Blood collection was performed in the early morning and during a clinical stability state (≥4 weeks after an exacerbation).
Intraclass correlation coefficient (ICC) (95%CI) was computed to assess eosinophilic counts stability, comparing baseline values with those recorded after 1, 3, and 6 months. Values ranging from 0.41 to 0.60 represented a moderate concordance, whereas those >0.60 a good concordance.11 Normal distribution of the variables was assessed by the Kolmogorov–Smirnov test. Pearson correlation coefficient was calculated. To assess the effect of the ICs on eosinophil counts changes, an ANOVA for repeated measures with the Bonferroni correction for multiple comparisons was performed. In 25 (7.8%) cases a multiple imputation method by chained equations was implemented under the assumption that missing data were missing randomly. A p-value <0.05 was considered statistically significant. Statistical computations were performed with the statistical package SPSS (version 20.0).
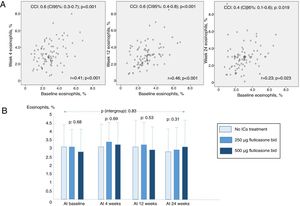
86 patients [mean (SD) age: 69.5 (8.9) years; 64% were women] were randomly selected. 21% were infected by Pseudomonas aeruginosa. At baseline mean (SD) FEV1 was 1403 (557) mL, and mean (SD) eosinophil percentage was 2.97 (1.3) %. A total of 74.4% and 41.8% patients had an eosinophil percentage of >2% and >3% at baseline, respectively, whereas 74.4% and 34.9% after 6 months of therapy. No statistically significant differences were found between the three groups.10
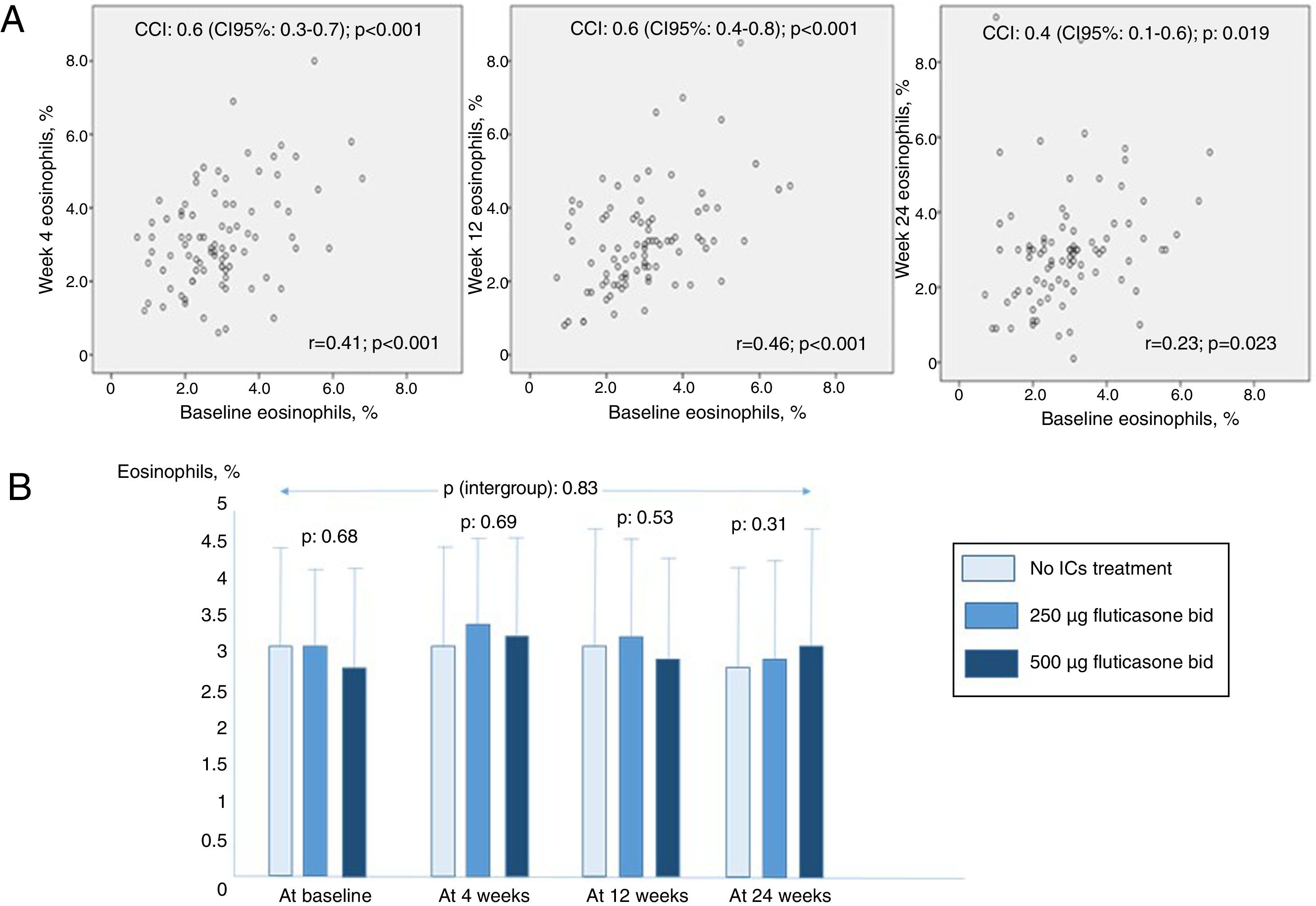
Fig. 1 shows higher ICC values in cases of closer measurements until 12 weeks, but lower in those at 24 weeks. Those results are confirmed by the correlation analysis showed in the same figure. 81% and 48.6% of patients did not change their eosinophil percentage >2% and >3% after 6 months, respectively. There were no differences between eosinophil percentage counts between groups at any time (p values between 0.31 and 0.69 at baseline, 4,12 and 24 weeks). Finally, it was not proved any significant effect of ICs treatment or its doses over time (intergroup p value: 0.83).
(A) Correlation graphs comparing 2-by-2 groups from circulating eosinophil baseline measures to 24-weeks follow-up measures. (B) Mean (standard deviation) eosinophils percentage counts at baseline, 4, 12 and 24 weeks in the studied groups. ICC: intraclass correlation coefficient; ICs: inhaled corticosteroids.
The results found in patients with bronchiectasis overlap those found in COPD patients. A study on 27,557 patients with stable COPD showed an ICC of 0.61.12 However, a post hoc analysis of TRISTAN proved variability between baseline and 24- and 52-week values.13 Moreover, in our study >80% of patients remained with >2% of eosinophils at 6 months of follow-up, but less than 50% remained with >3% compare with baseline data, as it was also found in COPD.14 Similarly, although circulating eosinophils seem to be a good biomarker for the assessment of the effectiveness of ICs in preventing exacerbations,3 their values is not affected by ICs in COPD patients.15 The results of this study highlight the necessity of performing repeated measures of eosinophils in the design of long-term studies to assess the role of this biomarker in clinical outcomes and treatment effectiveness in bronchiectasis.
In conclusion, moderate to good concordance between baseline and follow-up eosinophil counts was showed in patients with bronchiectasis. However, this concordance can decrease at six months but it is not affected by ICs treatment or its doses.
Previous presentationsThis data have not been presented in any scientific congress or meeting
FundingThis study has no funding.
Conflict of interestThe authors declare no conflict of interest.