The differential diagnosis for diffuse bilateral pulmonary micro nodules is extensive and typically includes infections, inflammatory disorders and malignancy. Meningothelial-like nodules of the lung, which are the result of proliferation of epithelioid cells within the interstitium, can also present as solitary pulmonary nodule, or as diffuse micro nodules, as in our patient. Although initially characterized as “minute pulmonary chemodectomas”, these nodules were subsequently found to lack neuroendocrine properties.1–4 The case adds to the growing literature on the natural history of pulmonary meningotheliomatosis (PM), as it remains an elusive clinical entity.
We report the case of a 58-year-old female, non-smoker, who presented to our clinic with an “abnormal” CT scan of the chest. The patient had presented to the emergency department a week previously for abdominal pain for which a CT abdomen/pelvis was obtained. The abdominal imaging was unremarkable except for lung nodules visualized at the bases. This prompted a dedicated chest CT scan and referral to our clinic. Her only symptom was intermittent dry cough for 3 months before presentation. Her physical examination, pulmonary function tests, routine blood work and rheumatology studies were unremarkable.
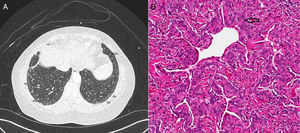
CT (computed tomography) scan chest showed multiple bilateral micronodules, some of which had ground glass appearance while others were more well-defined (Fig. 1A). Both upper and lower zones were involved although there was a basal predominance. Bronchoscopy with bronchoalveolar lavage (BAL) was unremarkable and showed normal macrophage predominance. Transbronchial biopsy was non-diagnostic. A video assisted thoracoscopic (VATS) biopsy was performed, the histopathology of which revealed multiple pulmonary meningothelial lesions (Fig. 1B). A final diagnosis of pulmonary meningotheliomatosis (PM) was made based on radiology and histopathological features.
(A) CT chest demonstrates multiple ground glass nodules along the subpleural area and lung periphery in the lower lobes bilaterally (marked with open arrows). (B) Histology on wedge biopsy demonstrates multiple minute pulmonary meningothelial-like nodules with intervening collagen (open arrow) (hematoxylin and eosin; 200×).
Meningothelial lesions of the lung were first described by Korn et al. in 1960, and were initially characterized as “minute pulmonary chemodectomas”.1 Since immunohistochemical studies showed it lacked neuroendocrine properties, the condition was renamed “minute pulmonary meningothelial-like nodules”.3,4 Other term that surfaced in our review of literature includes “diffuse pulmonary meningotheliomatosis”, particularly when there are numerous pulmonary micronodules causing symptoms.2,5 For purpose of uniformity, we will refer to this entity as “pulmonary meningotheliomatosis” (PM) henceforth.
Data from large retrospective studies so far show that it most commonly presents in the sixth decade of life.2,6,7 The age range can be between 20 and 80 years of age.6,7 The condition seems to have a strong female predilection.2,6,7 Although mostly diagnosed incidentally on pathology specimens, in patients with diffuse micronodules, non-specific symptoms (e.g.: cough, shortness of breath, fatigue) and pulmonary function abnormalities have been reported.2
PM can present as a solitary nodule, or more commonly as multiple, sub-centimeter, ground glass nodules on the CT scan.2,7,8 While majority of the cases have reported basal predominance, this does not seem to be universal.8 Although surgical lung biopsy seems to have a higher yield for diagnosis, transbronchial biopsy has also been utilized successfully.5,9 When it presents as diffuse micronodules, such as in our patient, it closely resembles more common etiologies such as granulomatous infections or metastatic malignancy, from which it needs to be distinguished. Therefore, given its rarity and lack of a characteristic radiological pattern, diagnosis of PM requires histopathology.
The etiology and risk factors for proliferation of meningothelial-like nodules remain unclear. It is more commonly associated with chronic lung insults than with acute lung injury.6 One of the most commonly reported association for meningothelial lesions has been with pulmonary thromboembolic disease.2 This was highlighted again in the retrospective study by Mukhopadhyay et al. where the highest incidence of meningothelial lesions was in patients with thromboembolic disease/infarcts (5/12; 42%).6 Interestingly, 26% of patients were also found to have smoking related interstitial lung disease such as respiratory bronchiolitis-associated interstitial lung disease/desquamative interstitial pneumonia.6 However, relationship with smoking remains unclear and causality cannot be inferred based on available data. Pulmonary meningothelial nodules have also been found in higher incidence in patients with malignant pulmonary tumors than in those with benign disease (7.3% versus 2.5%; P=.044).7 In the analysis of 121 patients by Mizutani et al., meningothelial lesions were found more often in patients with lung adenocarcinoma than with other primary pulmonary malignant tumors.7 A similar trend was noted in the study by Mukhopadhyay et al. but was not statistically significant.6 Thus, based on most of the studies, the meningothelial proliferation likely occurs in the setting of a chronic lung disease, as a reaction to hypoxia, ischemia or an underlying malignancy.
On histopathology, PM is characterized by an interstitial proliferation of epithelioid cells with oval, bland nuclei with stippled chromatin. These cells are arranged in nests within the alveolar septa, usually expanding it, and they are usually found around pulmonary veins.2 As they expand, they may connect to each other with intervening collagen.2 However, proximity to pulmonary veins is not universal.6 Immunohistochemical characteristics usually include immunoreactivity with antibodies to vimentin, epithelial membrane antigen (EMA) and progesterone receptors (PR).2,6 Niho et al. initially reported that about half of the pulmonary meningothelial nodules exhibited immunoreactivity for the PR.4 In the series by Mukhopadhyay et al., all the patients with pulmonary meningothelial lesions stained positive for PR.6 Interestingly, progesterone receptor positivity has also been identified in approximately 50–60% of patients with non-small cell cancer, particularly adenocarcinoma.10 Even though progesterone receptors are so far not a major therapeutic target in lung cancer, the higher incidence of progesterone receptor in meningothelial-like nodules and its potential co-existence with adenocarcinoma is noteworthy. This could also, in part, explain the increased incidence of meningothelial-like nodules in female gender. Staining with CD 56 is yet another immunohistochemical marker that was reported by Mukhopadhay et al.6 Presence of CD 56, although can be seen in neuroendocrine cells, its presence in meningiomas also confirms the meningothelial origin of these pulmonary nodules. While immunohistochemistry can aid in confirmation in ambiguous situations, a confident diagnosis can be made based on the histology pattern.7,9 Thus, PM which represents proliferation of epithelioid cells within interstitium, should be considered in the differential diagnosis of diffuse micronodules of the lung. However, its exact relationship with underlying lung diseases and natural history needs to be studied further.