Although modern anticancer therapies have significantly improved survival, interstitial lung disease (ILD) remains a critical determinant of short and long-term outcomes in cancer patients [1]. This is particularly evident in non-small cell lung cancer (NSCLC), where ILD may result in therapeutic interruption and indicates poorer prognosis [2]. ILD not only increases the risk of immune-related pneumonitis and radiation pneumonitis [3], but also represents a frequent complication of anticancer therapies in NSCLC [4]. This clinical challenge was highlighted in the TATTON trial, where the combination of osimertinib and durvalumab was discontinued due to ILD-related adverse events [5]. Notably, the concurrent use of immune checkpoint inhibitors (ICIs) with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGFR-TKIs) appears to exacerbate ILD risk. Existing literature further demonstrates the severity of ILD complications, with a reported 17.5% mortality rate among ILD patients receiving ICI therapy [6], accounting for 35% of anti-Programmed Death-1 (PD-1)/Programmed Death Ligand-1 (PD-L1) treatment-related fatalities [7]. Furthermore, NSCLC patients frequently require concomitant medications for comorbidities. However, the effects of these medications with anticancer therapies on ILD are still not well understood. These findings have raised concerns regarding ILD risk.
The American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) have established clinical guidelines for monitoring and managing pulmonary adverse events (AEs) related to cancer treatment [8]. Nonetheless, the specific risk of ILD associated with combination therapy remains poorly characterized, highlighting the importance of striking a balance between anticancer effectiveness and ILD reduction. Therefore, this study systematically evaluates the impact of concomitant medications and anticancer therapies on ILD progression in NSCLC patients, aiming to characterize its features and identify risk factors.
This observational, retrospective pharmacovigilance analysis was conducted using data from the FDA Adverse Event Reports System (FAERS) database between 2015 and 2023, utilizing preferred terms of ILD-related events from the Standardized Medical Dictionary for Regulatory Activities Queries (MedDRA Version26.0) for coding. ILD cases were identified using the following MedDRA preferred terms: idiopathic pulmonary fibrosis, interstitial lung disease, pulmonary toxicity, pneumonitis chemical, pneumonitis, pulmonary fibrosis, radiation pneumonitis [9–11]. We analyzed adverse event (AE) reports specific to NSCLC, categorizing patients by anticancer therapies and concomitant medications (including anti-thrombotics, thyroxine, calcium channel blockers, β-adrenergic receptor blockers, ACEIs/ARBs, gastric acid suppressants, diuretics, biguanides, hormonal agents, magnesium oxide, statins, amiodarone, opioid agents, methotrexate, and antibiotics). These medications were grouped as categorical variables. ILD-related events were identified as study outcomes. The time-to-event was calculated as the interval between the initiation of primary suspect (drug start date) and adverse event reporting date (ILD onset date). Primary suspect drugs were systematically identified through FAERS database records. Cases lacking drug start date were excluded to ensure temporal accuracy. Patient outcomes of adverse events were categorized by reporter according to standard FDA reporting system: death, life-threatening, hospitalization (initial or prolonged), disability, congenital anomaly, required intervention to prevent permanent impairment/damage, and other serious (important medical event). Cox proportional hazards regression models were used to evaluate associations between potential risk factors and ILD in NSCLC patients. The proportional hazards assumption was verified using Cox–Snell residuals, which assess the goodness-of-fit of survival models by evaluating whether the residuals follow a unit exponential distribution [12]. Variables with P<0.15 in univariate analyses were included in the multivariable model, with statistical significance set at P<0.05. Results are presented as hazard ratios (HR) with 95% confidence intervals.
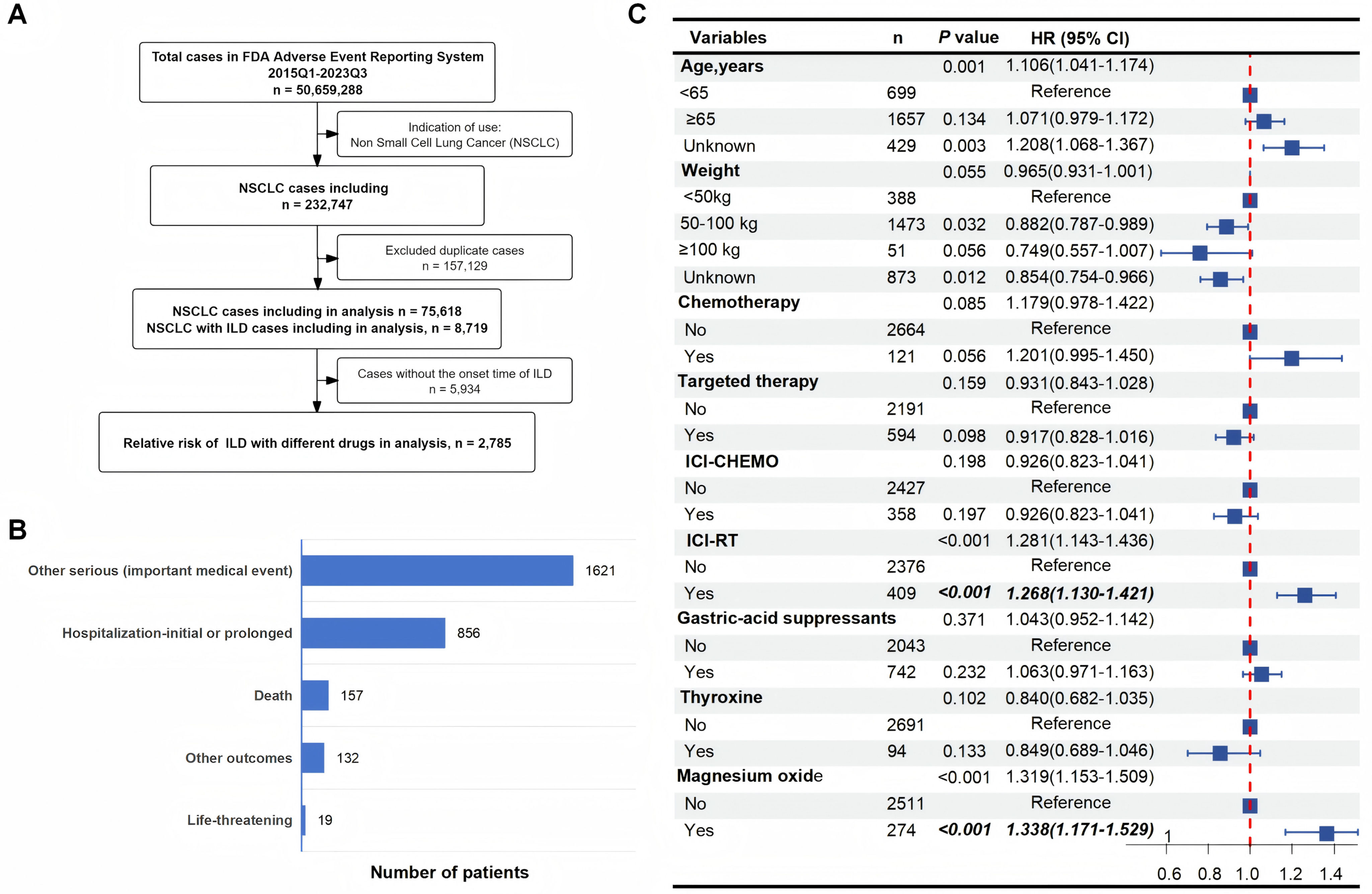
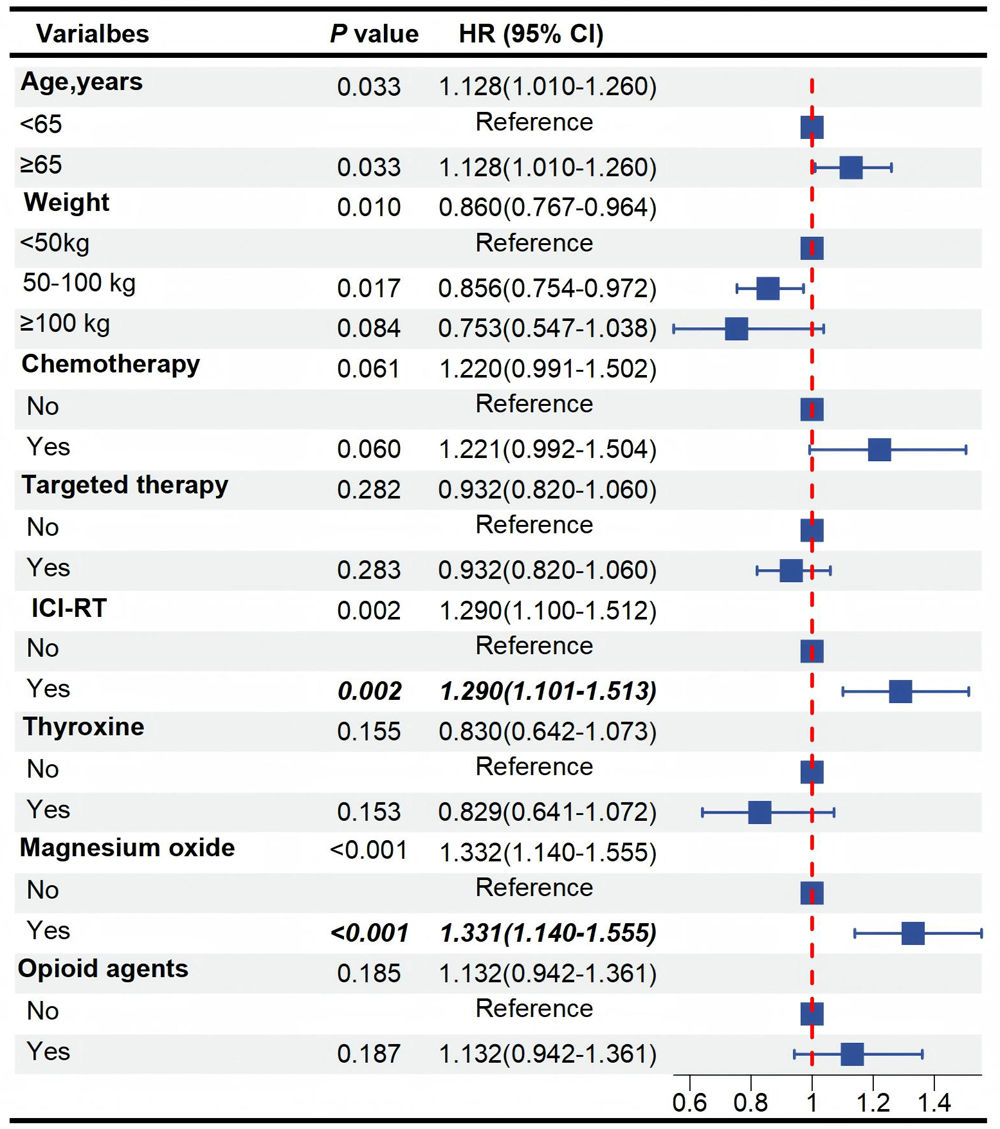
In this retrospective, observational study, we analyzed 75,618 NSCLC reports from the FAERS database, identifying 8719 ILD cases. After excluding 5934 cases lacking drug initiation dates, 2785 cases with complete documentation of both drug initiation and ILD onset dates were included for analysis (Supplementary Table 1, Fig. 1A). The incidence of ILD in NSCLC patients was 11.53%, with a median time to diagnosis of 34 days (IQR: 13–86). Patients over 65 years of age accounted for the highest proportion (59.5%) while 25.1% were under 65 years old. More than half (63.6%) of the case reports originated from Japan. Treatment-stratified analysis indicated that 1952 patients (70.1%) received ICI, 828 (29.7%) received targeted therapy, 409 (14.7%) received ICI combined with radiotherapy (ICI-RT). The commonly used concomitant medications for NSCLC patients were taken into consideration. In this study, the patient outcomes of reported ILD adverse events included other serious (important medical event) in 1621 cases (58.2%), hospitalization-initial or prolonged in 856 cases (30.7%), death in 157 cases (5.6%), life-threatening in 19 cases (0.7%), and other outcomes in 132 cases (4.7%) (Fig. 1B). Multivariate Cox regression analysis showed that ICI-RT combination therapy could be significantly associated with higher ILD reporting rates (HR 1.268; 95% CI 1.130–1.421; P<0.001). Notably, magnesium oxide administration could be independently associated with earlier ILD onset (HR 1.338; 95% CI 1.171–1.529; P<0.001). Detailed analytical results are presented in Fig. 1C and Supplementary Table 2. Subgroup analyses showed associations between magnesium oxide and ILD development across weight (50–100kg: HR 1.538, 95% CI 1.313–1.803; P<0.001), and males (HR 1.427, 95% CI 1.235–1.648; P<0.001) subgroups. These associations remained significant regardless of chemotherapy or targeted therapy, gastric-acid suppressants. Critically, magnesium oxide use could be associated with an elevated ILD risk in both opioid non-users (HR 1.373; 95% CI 1.193–1.581; P<0.001) and users (HR 1.382; 95% CI 1.011–1.889; P=0.042; P for interaction=0.842). Furthermore, this association persisted across age subgroups, demonstrating a higher but statistically correlation risk in individuals aged<65 years (HR 1.690; 95% CI 1.284–2.225; P<0.001) compared to those aged≥65 years (HR 1.319; 95% CI 1.126–1.545; P=0.001; P for interaction=0.345). These findings suggest an association between magnesium oxide use and increased reporting of ILD, which persisted regardless of concurrent opioid use or age differences (Supplementary Table 3). Collectively, our analysis characterized ILD cases and severe outcomes. Both ICB-RT combination therapy and magnesium oxide could be associated with increased risk of ILD. These findings provide valuable insights for further research and clinical practice in the management of NSCLC patients at risk of ILD.
Assessment of risk factors for interstitial lung disease (ILD) in non-small cell lung cancer (NSCLC). (A) Flowchart based on FAERS database in this study. (B) Distribution of ILD-related outcomes in NSCLC patients, presented as case numbers: other serious (important medical event), 1621 cases; hospitalization (initial or prolonged), 856 cases; death, 157 cases; other outcomes 132 cases; life-threatening 19 cases. (C) Multivariate Cox proportional hazards model for onset time associated with ILD. NSCLC, Non-Small Cell Lung Cancer; ILD, Interstitial Lung Disease; RT, Radiotherapy; ICI, Immune Checkpoint Inhibitors; CHEMO, Chemotherapy; HR, Hazard Ratio; CI, Confidence Interval.
Contemporary anticancer therapies have markedly improved survival outcomes. However, anticancer therapies concomitantly induce significant pulmonary toxicity. ILD remains a critical complication in NSCLC patients, significantly influencing treatment decisions, patient survival, and quality of life. The observed 11.53% incidence of ILD in our cohort corroborates with real-world data from Suzuki et al., who reported a 14.5% incidence associated with ICIs, which is approximately threefold higher than clinical trial estimates [4]. This may be partly explained by the substantial proportion of Japanese cases, a population with documented genetic susceptibility to ICI- and EGFR-TKI-related ILD. This includes non-HLA variants (C22orf34, TSHZ2) and HLA variants (DRB1*04:05) [4,10,13]. The median onset time of 34 days (IQR 13–86) highlights the clinical significance of this time window for enhanced monitoring. Furthermore, 5.6% of ILD cases resulted in death, while 58.2% were classified as other serious events. These data align with prior reports of severe ILD presentations in Japanese cohort [4]. ICI monotherapy (35.5%) or ICI-RT (14.7%), aligns with recent studies. A study conducted on lung cancer patients showed that postoperative AE-ILD had a mortality rate of 43.9%, further emphasizing the severity of ILD in this population. Previous research has shown that 35% of patients receiving immunotherapy develop ILD, with a mortality rate of 17.5% among affected individuals [4,10]. This suggests a correlation with our observation that ICI-RT could be associated with increased risk of ILD, reinforcing concerns over combined-modality toxicity. These findings emphasize the necessity for standardized assessment, systematic monitoring, and optimized management in NSCLC patients receiving these therapies.
The identification of magnesium oxide as an independent risk factor for ILD suggests a clinically significant drug-disease interaction requiring careful consideration. This finding presents a clinical conundrum, given that magnesium oxide remains current guideline-recommended treatment for chronic constipation in adults [14]. Notably, more than 30% of lung cancer patients experience constipation, with incidence rates rising significantly among patients receiving opioid analgesics. This pattern substantially elevates magnesium oxide administration in cancer patients. While concomitant medications showed no significant associations, magnesium oxide exhibited a correlation with ILD risk across subgroups. Given the known risks of combination drug-induced ILD, particularly dose-dependent and synergistic effects [2,15], clinicians should exercise heightened clinical vigilance. The potential pulmonary effects of magnesium oxide represent a novel finding that warrants further investigation. Based on existing literature, we hypothesize that this signal may involve antacid-mediated gut microbiota alterations [16], with potential gut-lung axis modulation [17,18], or hypermagnesemia-related respiratory effects [19]. As a hypothesis-generating observation, these potential mechanisms require validation through prospective clinical studies and experimental models to establish any biological relevance. However, these hypotheses need to be tested in prospective clinical studies and animal models. Data on pulmonary adverse effects of magnesium oxide from available clinical trials remain limited, and this finding may be considered as hypothesis-generating for future research.
Given the widespread clinical application of magnesium oxide in cancer patients, these findings highlight the importance of recognizing its potential pulmonary risks, particularly in NSCLC patients receiving ICI-RT. Our prior work highlighted thyroxine for alleviating lung fibrosis induced by ICI-RT [20]. This study identifies constipation medications as potential risk factors for ILD progression in NSCLC patients, underscoring the impact of concomitant medications on ILD. However, a key limitation of this study is the reliance on MedDRA-coded ILD diagnoses. While the FAERS database provides a valuable platform for large-scale pharmacovigilance analyses using standardized terminology, the absence of detailed clinical information significantly constrains both the evaluation of pre-existing disease contributions and the accurate characterization of ILD severity. Additionally, this time-to-event analysis of ILD was limited to FAERS cases with complete documentation of both drug initiation and ILD onset dates. These limitations were partially mitigated by our stringent case selection criteria and sensitivity analyses. Nevertheless, future prospective studies incorporating imaging and multidisciplinary evaluation would be valuable to further investigate these pharmacovigilance signals. Additional limitations of this study include the absence of drug interaction analysis and mechanistic animal investigations. These limitations highlight the necessity of prospective clinical-basic science studies to fully delineate risk factors of ILD and mechanisms in NSCLC patients.
In summary, ILD critically impacts treatment outcomes in NSCLC patients. The ICI-RT combination therapy and magnesium oxide could be associated with an increased risk of ILD reporting in this study, reinforcing concerns about potential pulmonary toxicity. Our findings provide novel evidence regarding risk factors for ILD, emphasizing the need for further investigation of drug-induced mechanisms.
Author contributionsYM: data curation and wrote the manuscript. DCW: data curation and analyzed the data. QS, JCM, and AMJ: data analysis. DWC: review and conceptualization. JMY: conceptualization, supervision, and review.
Ethics approval and consentThe ethical approval is not available. The data from FDA Adverse Event Reporting System were obtained from publicly accessible database, and the requirements for obtaining informed consent and institutional review board approval were waived.
Consent for publicationNot applicable.
Artificial intelligence involvementNone of the material was produced with the assistance of any artificial intelligence software or tools.
FundingNot applicable.
Conflict of interestThe authors declare no competing interest.
Availability of data and materialsThe data underlying this article were accessed from FDA Adverse Event Reporting System. The derived data generated from FAERS database in this research are available from the corresponding author on reasonable request.
We are grateful to the work of FDA.