As we look toward the future of sleep apnea research, it is essential to acknowledge the pivotal contributions that have shaped the field to date while focusing on the innovations and paradigms that will drive its next chapter. Sleep-disordered breathing (SDB), long underdiagnosed and misunderstood, has moved to the forefront of discussions around systemic health, and its evolution reflects both technological advances and growing public health awareness. Increasingly, sleep apnea is recognized not merely as a sleep disorder but as a multifactorial condition with profound implications for cardiovascular, metabolic, cognitive, and emotional health.
The terms obstructive sleep apnea (OSA) and SDB were coined in the 1970s, but there were no formal estimates of their prevalence, although, based on their clinical experience, some researchers at the time thought that OSA might affect a notable subset of middle-aged adults. Initial studies in the 1990s suggested a prevalence of 4% but later studies started to indicate a much greater prevalence [1]. In the landmark São Paulo Epidemiologic Sleep Study (EPISONO), Tufik et al. reported a 32.9% prevalence of OSA among the adult population of the city of São Paulo, Brazil, reflecting how evolving methodologies and broader diagnostic awareness have reshaped our understanding of the condition's true burden [2]. Subsequent global analyses confirmed the magnitude of the issue. A pivotal study published in The Lancet Respiratory Medicine estimated that approximately 936 million adults worldwide suffered from some degree of OSA, defined as an apnea hypopnea index (AHI) of 5 or more events per hour combined with relevant symptoms or comorbidities, and that 425 million had moderate to severe OSA, defined as an AHI of 15 or more events per hour [3]. Building on this paradigm, another study using data from the EPISONO cohort investigated the prevalence of upper airway resistance syndrome (UARS) [4]. Unlike OSA, UARS is characterized by subtle inspiratory flow limitations that provoke sleep arousals without fulfilling the formal criteria for apneas or hypopneas [4]. To accurately identify UARS in the population, the study applied stringent diagnostic criteria, including an AHI of fewer than 5 events per hour, minimum oxygen saturation (SpO2) of at least 92%, the presence of inspiratory flow limitation during 5% or more of total sleep time, and daytime symptoms, such as fatigue or sleepiness. The prevalence of UARS was found to be 3.1% [4]. The condition was significantly more prevalent in women (4.4%) than in men (1.5%), suggesting a possible sex-related susceptibility [4]. Although the definition and even the existence of UARS remain subjects of ongoing debate in sleep medicine, these population-based findings provide empirical support to its clinical relevance. Importantly, they highlight the need to look beyond hypoxic thresholds and incorporate both physiological subtleties and subjective experiences into the diagnostic process. In this context, it may be increasingly useful to conceptualize UARS and OSA not as distinct entities but as part of a continuum of sleep-related breathing disorders [5]. Just as OSA is currently stratified by severity (mild, moderate, severe), UARS may represent an earlier or less overtly hypoxic manifestation within the same pathophysiological spectrum. This continuum model emphasizes the progression from flow limitation and arousals to more overt apneic events, suggesting the need for a more integrative framework for diagnosis, risk assessment, and early intervention.
This broader perspective is especially critical given that SBD, like OSA and UARS, remain markedly underdiagnosed worldwide. Barriers, including limited access to specialized care, low recognition of symptoms (especially among women), and reliance on narrow diagnostic criteria contribute to the underdetection of these conditions within healthcare systems. As a result, millions of people experience impaired quality of life, excessive daytime sleepiness, and increased cardiometabolic risk without receiving the care they need [3].
All of these factors must be taken into account when considering future strategies in respect of diagnosis and treatment. The heterogeneity of sleep-disordered breathing, the historical underdiagnosis of its milder forms, and the importance of individualized symptom profiles must inform the next generation of research and clinical practice. Today, the field stands at the threshold of a new era. Artificial intelligence, wearable technology, and precision medicine are reshaping the way sleep apnea is studied, diagnosed, and managed. Multi-omics approaches, which integrate data from genomics, metabolomics, and microbiome analysis, promise to refine phenotyping and lead to targeted therapies tailored to individual biological profiles.
Biomarker discovery and application will be central to this transformation. Molecular biomarkers, including specific inflammatory cytokines, oxidative stress markers and genomic signatures, are being investigated for their potential to signal early disease, stratify risk and predict treatment response. Machine learning models are being developed to interpret complex polysomnographic data and may soon assist in the production of personalized treatment strategies, including continuous positive airway pressure therapy, mandibular advancement devices, and neurostimulation. In respect of consumer-grade wearable devices, although they are not yet capable of providing clinically validated sleep staging, their ongoing evolution holds great promise for real-time tracking, early screening, and long-term monitoring.
Notable examples of wearable devices already approved by the U.S. Food and Drug Administration (FDA) include the Dreem 3S, a headband-based EEG device designed for high-fidelity home sleep monitoring [6]. In 2024, the FDA authorized a Predetermined Change Control Plan for the Dreem 3S, allowing continuous algorithm updates for sleep staging without the need for new regulatory submissions. Similarly, the Samsung Galaxy Watch received FDA clearance for its sleep apnea detection feature, enabling users to monitor signs of moderate to severe OSA using photoplethysmography signals. Most recently, the Apple Watch was also granted FDA authorization for a comparable function, expanding the availability of accessible screening tools for sleep-disordered breathing.
These tools may help to increase access to sleep health assessment in underserved populations and reduce the burden on sleep laboratories, which often have limited availability and accessibility. By integrating high-resolution physiological data with behavioral and molecular information, the field can move closer to a truly personalized approach that addresses not only the severity of respiratory events but also the unique biological and psychosocial contexts in which they occur. These advances represent a significant step forward in democratizing sleep diagnostics, addressing the longstanding issue of underdiagnosis, and creating new opportunities for early intervention and tailored therapeutic strategies.
Another particularly promising direction involves the identification and clinical application of OSA phenotypes. Historically treated as a homogeneous condition, it is now clear that OSA encompasses a range of pathophysiological mechanisms, symptom profiles, and treatment responses. Phenotypes may include positional OSA, REM-related OSA, insomnia with OSA (COMISA), and minimally symptomatic individuals with severe respiratory disturbances [7]. Furthermore, traits consisting of upper airway collapsibility, arousal threshold, loop gain and neuromuscular responsiveness offer deeper insight into the biological variability among patients. Recognizing these phenotypic differences is essential for advancing personalized therapy and optimizing treatment outcomes. It also creates the possibility for more targeted use of existing therapies and the development of new ones based on underlying mechanisms.
While imaging has traditionally played a limited role in the routine evaluation of sleep apnea, its future contributions may be more substantial, particularly in the context of personalized care [8]. Advances in imaging techniques, such as magnetic resonance imaging, computed tomography and positron emission tomography offer new opportunities to characterize the anatomical, functional and even inflammatory aspects of sleep-disordered breathing. For example, imaging may help identify patients with specific patterns of upper airway collapsibility, craniofacial structure, or neurocognitive impact, thereby informing more tailored therapeutic decisions. Emerging techniques, including dynamic airway imaging and multimodal neuroimaging, may also shed light on underexplored dimensions of the disorder, particularly central nervous system involvement and treatment responsiveness.
At the same time as these new tools emerge, sleep apnea research must remain grounded in the broader social realities that shape who gets diagnosed and treated. Social and economic shifts, like the rise of the “gig economy”, irregular shift work, 24/7 work cultures, the expansion of urban peripheries, long commutes, and economic precarity have disproportionately affected sleep health among lower-income populations. These changes have deepened sleep inequities and brought into focus the need for scalable, accessible diagnostic tools and interventions that account for social determinants of health. Community-based screening initiatives, mobile health units, and telemedicine platforms may help to bridge this gap. However, their success will depend on rigorous validation, regulatory oversight, and a commitment to ensuring access across diverse populations as well as understanding how digital divides, data privacy concerns, and insurance systems can create new barriers to care if not proactively addressed.
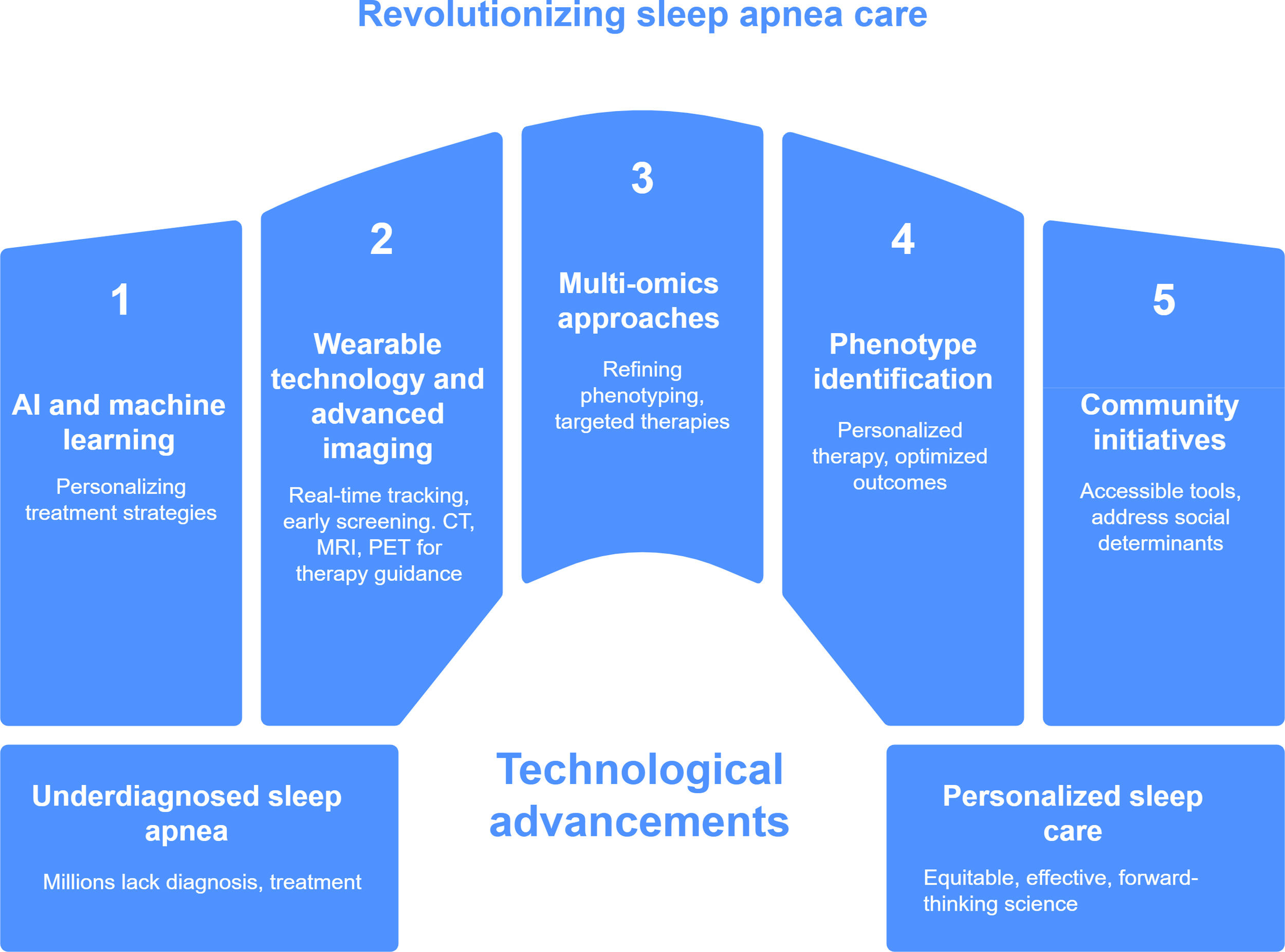
As we move forward, aligning scientific innovation with social responsibility will be crucial for transforming sleep apnea care into a model of precision medicine and public health integration (Fig. 1). The challenge is not simply to build better tools, but to ensure that these tools reach the people who need them most, regardless of geography, income, or background. The next frontier in sleep apnea research will depend on interdisciplinary collaboration among clinicians, engineers, data scientists, public health leaders, and patient communities. Only through such concerted efforts can we unlock the full potential of a more just, effective, and forward-thinking science of sleep.
Revolutionizing sleep apnea care through technological innovation. This figure illustrates key pillars driving the transformation of sleep apnea diagnosis and management. The central theme highlights how technological advances are bridging the gap between widespread underdiagnosis and the implementation of personalized, equitable care. The five major components include: (1) artificial intelligence and machine learning, enabling personalized treatment strategies; (2) wearable technology for real-time monitoring and early screening, now integrated with advanced imaging tools, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) to support anatomy-based treatment decisions; (3) multi-omics approaches to refine phenotyping and support targeted therapies; (4) phenotype-based identification to guide individualized therapy and improve outcomes; and (5) community-based initiatives focused on accessibility and addressing social determinants of health. Together, these innovations aim to shift sleep apnea care toward a more inclusive, data-driven, and patient-centered model.
All authors made substantial contributions to collecting, conceptualizing, drafting, reviewing, and editing the review article and constructive guidance for the final version of the article. All authors read and approved the final manuscript.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Conflict of interestThe authors have no competing interests to disclose.
Data availability statementData sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the Associação Fundo de Incentivo à Pesquisa and grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, recipient MLA: 2020/13467-8), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, recipient: MLA and ST).