Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive disease characterized by worsening dyspnea and irreversible loss of lung function.1 Prognosis is poor, with an estimated median survival of 2–4 years.2,3 Acute exacerbations of IPF (AE-IPF) are defined as episodes of acute, clinically significant respiratory deterioration, characterized by evidence of new widespread alveolar abnormalities, and the exclusion of infection or other potential triggers is no longer required, since the new diagnostic criteria published in 2016.4 AE-IPF prognosis is poor, with a median survival of 3–4 months and current evidence suggests that almost half of IPF deaths are associated with AE-IPF.4–7 Studies have shown that initiation and worsening of IPF may be associated with viral, bacterial and fungal infections and/or an altered lung microbiome.8–19
This was a multicenter prospective study, carried out on a cohort of patients admitted for AE–IPF and conducted at five centers in Spain. The recruitment period lasted for one year and follow up was also one year. The aim was investigating the association between acute exacerbation and respiratory infection and identifying potential predictors of mortality.
The protocol was approved by the institutional review board at each site (PEIBA 1593-N-17) and all patients provided written informed consent.
Patients were eligible if they were: aged 30–90 years, diagnosed with IPF by a multidisciplinary board,1 and admitted with an AE-IPF. Other interstitial lung diseases or admissions for non-respiratory causes were excluded.
All subjects underwent an exhaustive evaluation for respiratory infections: blood cultures, sputum mycobacterial culture and Gram stain, bacterial culture of bronchial aspirate (BAS) and/or bronchoalveolar lavage (BAL), sputum fungal culture, urinary antigen tests (Streptococcus and Legionella), pneumonia serological tests (Legionella, Mycoplasma, Chlamydophila, Coxiella and Brucella), viral serologies (respiratory syncytial virus, influenza A and B, parainfluenza, adenovirus, cytomegalovirus (CMV), herpes simplex (HSV), and varicella-zoster), detection by real-time reverse transcription-PCR (RT-PCR) of influenza virus in nasopharyngeal aspirates, and bacterial culture and smear microscopy from pleural fluid (if pleural effusion). Determinations were performed within the first 48h after admission and, whenever possible, before the start of empirical antibiotic treatment.
Pneumonia was defined as a consolidation on chest X-ray or computed tomography (CT) scan with at least one of the following: fever, productive cough, or leukocytosis/neutrophilia. When ground glass opacities were observed with no evidence of infection or other cause, it was considered an idiopathic exacerbation.
To assess functional impairment, all patients had at least one spirometry in the year prior to admission and another within 3–6 months after exacerbation.
The significance level was established at a value of α=0.05.
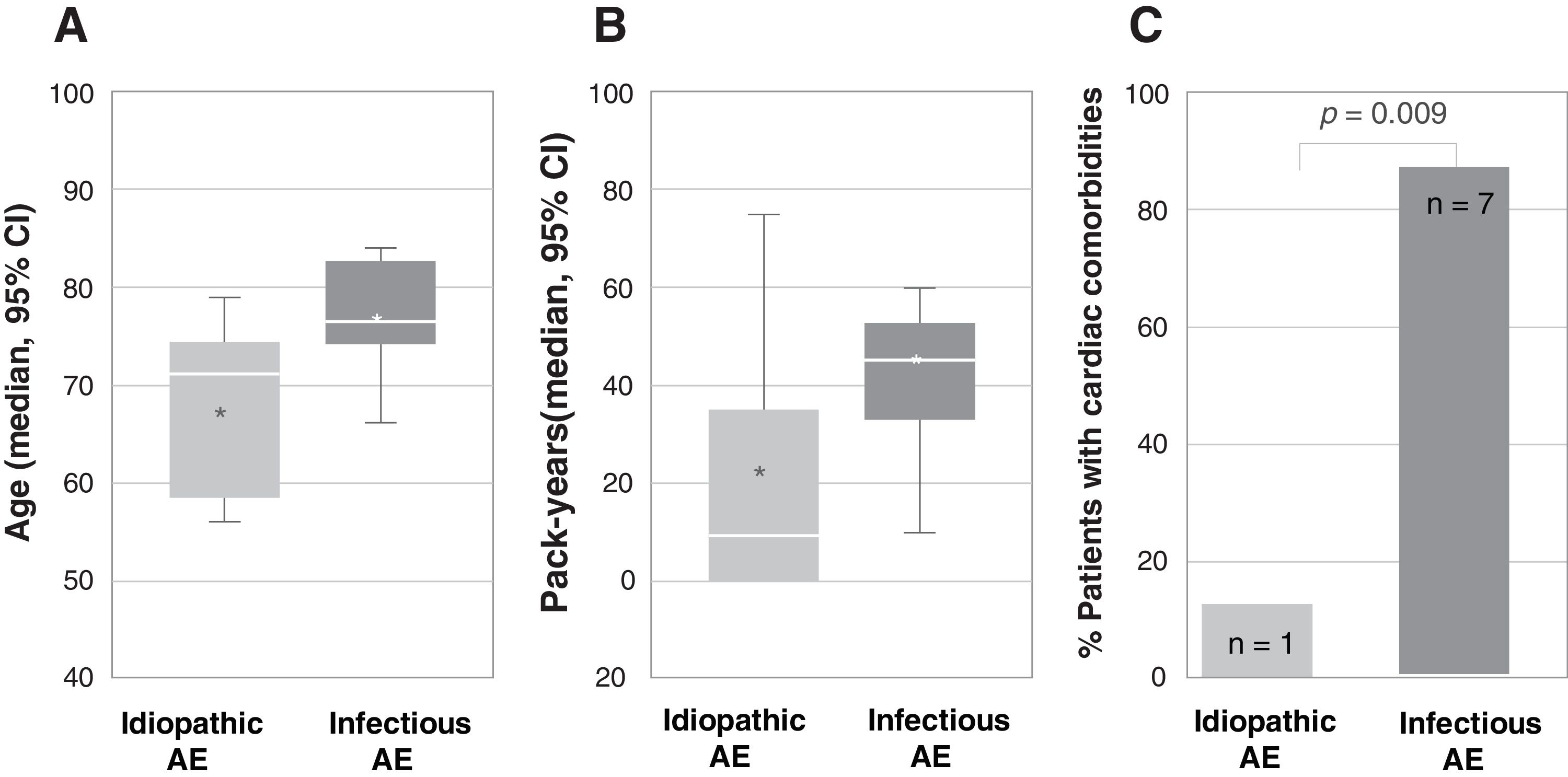
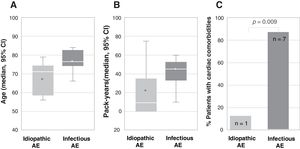
From April 2018 to March 2019, 23 patients with AE-IPF were enrolled. Mean age was 70.5, 62.5% were 70 or older, 82.6% were male and 69.5% were active or former smokers. Respiratory infection was listed as the possible triggering cause of AE-IPF in 34.8%, while in the remaining, no other causes, such as pulmonary embolism (PE), ischemic heart disease or heart failure were identified. More patients in the group of infection-triggered AE-IPF were older (77 vs. 67 years, p=0.006), and had a higher smoking load (45.5 vs. 22.6 pack-years, p=0.019) and cardiac comorbidities (87.5% vs. 12.5%, p=0.009) (Fig. 1).
Association between type of acute exacerbation, infection-triggered or idiopathic, and (A) age (represented as median age in years and CI 95%; p=0.006), (B) smoking load (represented as median number of pack-years and CI 95%; p=0.019) and (C) cardiac comorbidities (represented as percentage of patients with cardiac comorbidities; the number of patients is indicated within each bar; p=0.009). In figures A and B, the bottom and top of the boxes span the 25th (Q1) and 75th (Q3) percentiles of data points, the horizontal bar within the boxes indicates the 50th percentile (Q2, median), the whiskers indicate 1.5 times the interquartile range (Q3–Q1), and the asterisks indicate the mean. AE, acute exacerbation.
Among the 16 patients (69.6%) who were on antifibrotic therapy (14 pirfenidone and 2 nintedanib), the median time from diagnosis to AE-IPF was 26.1 months. No association was found between the type of acute exacerbation, infection-triggered or idiopathic, and the antifibrotic treatment received.
On high-resolution computed tomography (HRCT), a typical usual interstitial pneumonia (UIP) pattern was predominantly observed in 60.9%, while 30.4% had a possible UIP pattern and 8.7% an inconsistent UIP pattern. The radiological patterns were not associated with pulmonary infection (p=0.094) or mortality (p=0.730). The predominant radiological finding at admission was ground glass opacity (GGO) (69.6%), followed by diffuse alveolar damage (DAD) (21.7%) and consolidation (8.7%). Again, no association was found between the type of acute exacerbation and the radiological findings. 21.7% were diagnosed with community-acquired pneumonia (CAP): 13.0% bilateral pneumonia, 4.3% unilateral bilobar pneumonia and 4.3% unilobar pneumonia.
Almost half of the population included (47.8%) had some form of cardiovascular disease: 21.7% arterial hypertension, 13.3% atrial fibrillation, 8.7% ischemic heart disease, and 4.3% stroke. Regarding concomitant respiratory disease: 21.7% had emphysema, 21.7% pulmonary hypertension (PHT), 4.3% sleep apnea–hypopnea syndrome (SAHS), and 2.3% long-standing PE. Based on functional tests, 39.1% had mild IPF (FVC≥80%) at admission, while 60.9% had moderate or severe disease (FVC<80%).
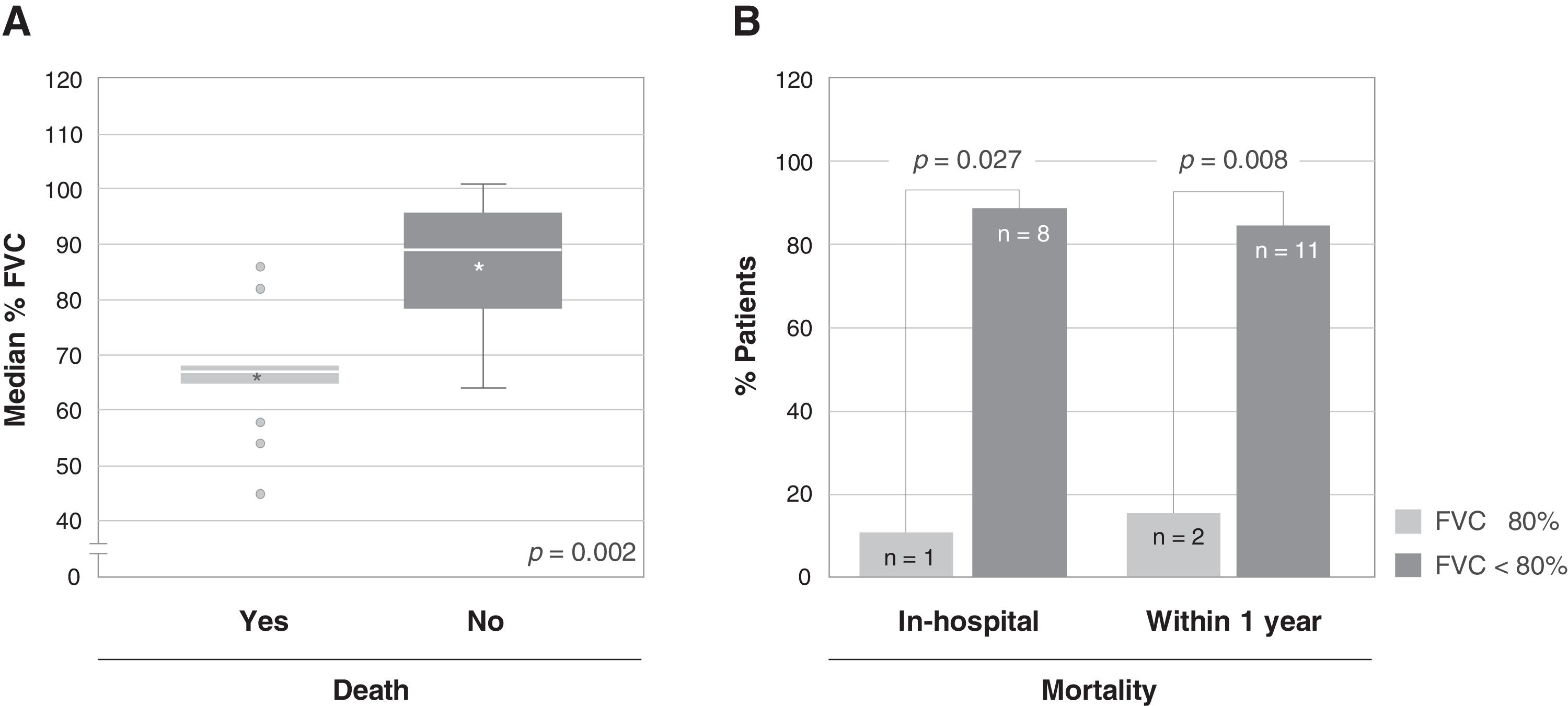
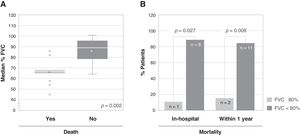
A total of 13 patients (56.5%) died due to an acute exacerbation, of whom 9 (69.2%) died during hospital stay. Among the variables analyzed as possible predictors of mortality, a statistically significant association was only found between the severity of the disease (%FVC) and mortality, both in-hospital (11.1% vs. 88.9%, p=0.027) and after one year (15.4% vs. 84.6%, p=0.008) (Fig. 2). The patients who died had a significantly lower FVC (66.08%) than the patients who survived (85.90%). There was no significant association between the diffusing capacity for carbon monoxide (DLCO) and mortality, although DLCO was lower in the group of patients who died (28.63%), compared to the patients who survived (35.71%).
Association between the severity of the disease and mortality. (A) Association between the median percentage of forced vital capacity (FVC) of patients at admission and death after one year from admission. The bottom and top of the boxes span the 25th (Q1) and 75th (Q3) percentiles of data points, the horizontal bar within the boxes indicates the 50th percentile (Q2, median), the whiskers indicate 1.5 times the interquartile range (Q3-Q1), the asterisks indicate the mean, and the grey circles the outliers. (B) Association between the severity of the disease, expressed as percentage of FVC (FVC≥80% vs. FVC<80%), and mortality, both in-hospital (left, p=0.027) and within one year from admission (right, p=0.008); the number of patients is indicated within each bar.
Other possible predictor of mortality could be the severity of respiratory failure at admission, but unfortunately data regarding oxygen requirements and type of respiratory support needed were not collected in all patients.
No significant differences in survival were found depending on whether patients were on antifibrotic treatment or not. We believe those differences were probably not detected because of the small sample size.
An analysis of the number of admissions throughout the year showed that they were more frequent during winter and spring (34.8% and 39.1%), as was the development of infection-triggered acute exacerbation (50% and 37.5%). No infection-triggered acute exacerbations were registered during the summer. Despite the fact that all hospitalized patients had received influenza vaccination, 13.04% were found to be positive for influenza and treated with oseltamivir. Influenza viruses were the viruses found most frequently in our study population, which further supports the role of seasonal epidemics in triggering AE-IPF.17,20
Our study has some limitations. It is possible that a number of cases of acute exacerbation considered idiopathic actually had an infectious origin that could have gone unnoticed, because viral detection by PCR was not performed in all patients and it was only used for influenza viruses. In addition, we only have BAL data of two patients, with negative bacterial culture results, and viral detection and BAL cellularity were not measured. On the other hand, our results show no differences in the survival of IPF patients due to either an acute exacerbation caused by infection or an idiopathic event.
In summary, our study demonstrates the importance of respiratory infection as a cause of exacerbation of IPF, especially due to influenza during winter and spring. These results emphasize the high mortality rate of acute exacerbations and confirm the predictive value of baseline FVC prior to admission, with patients with lower FVC values being at higher risk of death. In this context, an acute exacerbation may be the cause of a new diagnosis of IPF. Finally, patients with the greatest probability of having acute exacerbations due to infection are those of older age, with a higher smoking load and associated cardiovascular comorbidities.
Authorship contributionsNFR conceived and designed the study. NFR and ADRO contributed to the writing of the manuscript. NFR, ADRO, BMJR, ALB, JARP, ZPH, MFG, PGZ, MPM and MER participated in the analysis and discussion of the data, revised the article critically and approved the final version.
Support statementThis study was funded by Fundación Neumosur, Sevilla, Spain (Grant No. 13/2017), who provided financial support for the conduct of the research and had no other involvement either in the preparation of the article, the study design, the collection, analysis and interpretation of data, writing of the report or in the decision to submit the article for publication.
Conflict of interestDr. Fouz-Rosón reports grants from Fundación Neumosur, Sevilla, Spain, during the conduct of the study. The authors declare no other conflicts of interest relevant to this manuscript.
We thank Drs. Alicia Cortés-Caballero, Alicia Padilla-Galo, Antonia Soto-Venegas and Luis Fernández de Rota for data collection. Medical writing support was provided by Luis F. García-Fernández, PhD (Medical Statistics Consulting, S.L., Valencia, Spain).