Cough hypersensitivity is a pathophysiological feature in chronic cough.1 Chemical cough challenge tests, using agents like capsaicin and citric acid, have been widely utilized to evaluate cough hypersensitivity.2 However, chemical stimulation-related cough challenge test cannot totally assess the condition of cough hypersensitivity. A more comprehensive assessment of cough sensitivity is needed. Previous studies showed that patients with idiopathic pulmonary fibrosis or upper respiratory tract infection showed enhanced cough sensitivity to vibration on chest or airway.3,4 Additionally, mechanical stimulation of the external auditory canal (Arnold's nerve reflex) can trigger cough responses in chronic cough patients.5,6 Recent evidence shows that mechanical stimulation at specific somatic points helps identify chronic cough patients with poor responsiveness to guideline-based therapies.7 These findings underscoring the importance of mechanical stimulation-related cough sensitivity (MCS) assessment. Moreover, chronic cough can be categorized into different clinical phenotypes.8 The differences in MCS among different clinical phenotypes of chronic cough still need further investigation. Herein, we aimed to: (1) clarify the relationship between mechanical and chemical stimulation cough sensitivity, (2) characterize different phenotypes of cough hypersensitivity, and (3) elucidate the potential significance of cough sensitivity assessment in distinguishing the clinical phenotypes of chronic cough.
The research process is presented in Appendix Fig. S1. The tracheal vibration test (TVT) was used to assess MCS, while capsaicin cough challenge tests evaluated chemical stimulation-related cough sensitivity (Appendix Material S1). Mechanical stimulation-related cough sensitivity is classified as heightened (MCS+) if cough or urge or cough occurs during TVT, and non-heightened (MCS−) otherwise. Patients with chronic cough (duration of >8 weeks) and healthy subjects were recruited from the First Affiliated Hospital of Guangzhou Medical University from 2019 to 2023 (Appendix Material S2). The details of diagnosis and treatment for different clinical phenotypes of chronic cough were presented in Appendix Material S3. All subjects underwent baseline TVT and capsaicin challenge tests, with patients completing visual analog scale (VAS) and Leicester Cough Questionnaire (LCQ). Post-treatment evaluations repeated TVT, capsaicin challenges, VAS, and LCQ. Participants unable to attend on-site follow-up visits underwent structured telephone-based assessments. All participants signed the consent form before entering the study. The ethics statements have been placed in the First Page file only, as required by the editors.
Statistical analyses were performed using SPSS 26.0. Continuous variables with normal distribution were presented as mean±standard deviation; otherwise expressed as median (the 25th and 75th percentiles). Categorical variables were described as numbers (percentages). Parametric (t-test, ANOVA) and nonparametric (Mann–Whitney U, Kruskal–Wallis, Wilcoxon, Friedman's) tests were applied to continuous data as appropriate. For categorical variables, Pearson's Chi-Squared test, Fisher's exact test, McNemar's exact test or Cohen's kappa coefficients was applied as appropriate. Univariate and multivariate logistic regression analyses were conducted to explore risk factors associated with Gastroesophageal reflux-related cough (GERC).
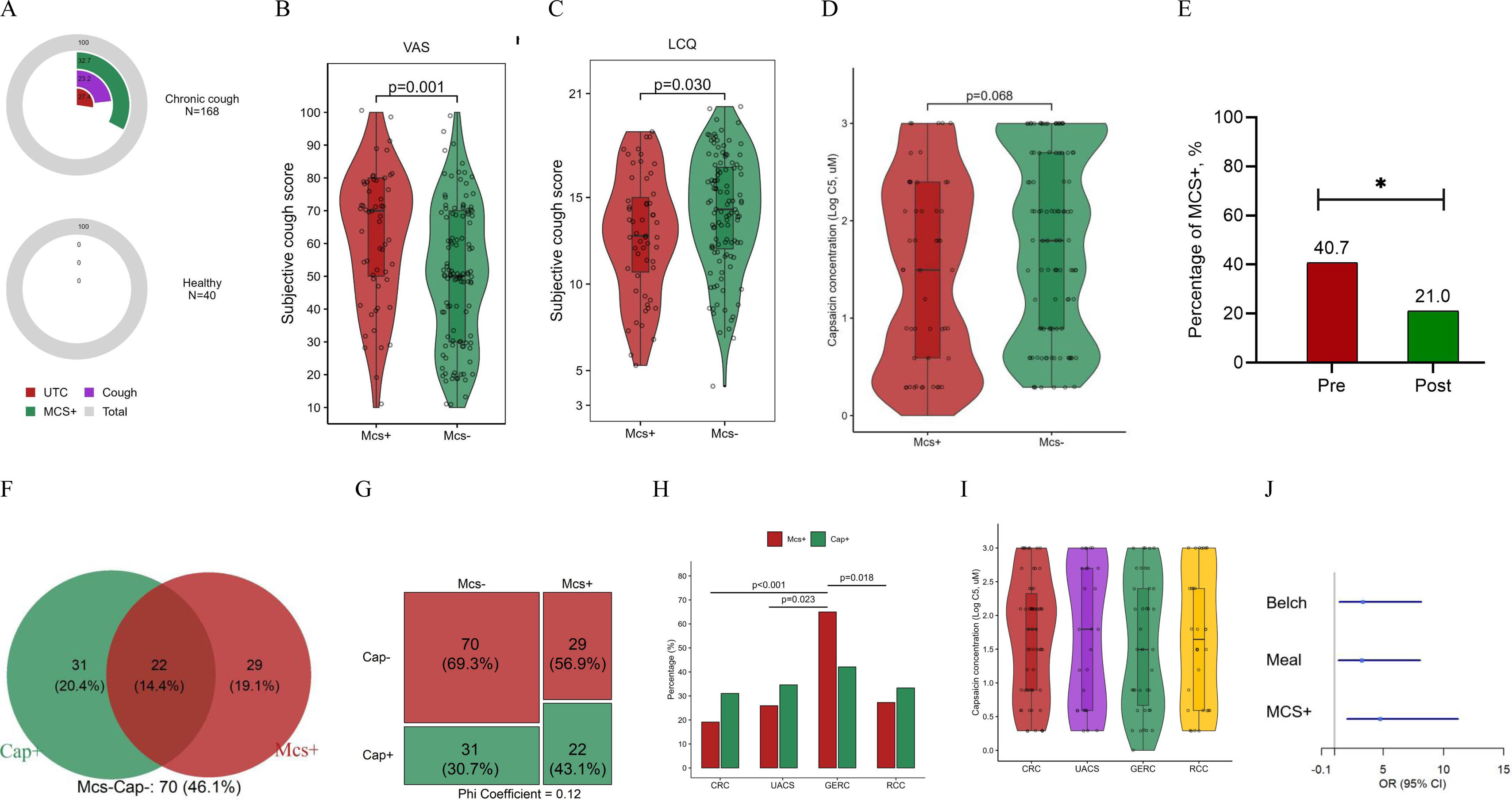
A total of 168 patients with chronic cough and 40 healthy subjects were enrolled (Appendix Table S1). The incidence of MCS+ in patients was significantly higher than in healthy subjects (32.7% vs. 0%, p<0.001, Fig. 1A). Between MCS+ and MCS− subgroups, no difference in age, body mass index, cough duration or gender distribution were observed (Appendix Table S2). The MCS+ group had significantly higher VAS scores and lower LCQ scores compared to the MCS− group (Fig. 1B & C). There was no significant difference in LgC5 (log-transformed capsaicin concentration inducing 5 coughs) between the MCS+ (1.49 [0.59, 2.40]) and the MCS− (1.80 [0.89, 2.70]) (p=0.068, Fig. 1D). After treatment, the incidence of MCS+ was significantly decreased (40.7% vs. 21.0%, p=0.024) (Fig. 1E).
The mechanical and chemical stimulation-related cough sensitivity in chronic cough. (A) The prevalence of positive response to tracheal vibration test (TVT) in patients with chronic cough and healthy subjects. (B) The visual analog scale (VAS) between MCS+ and MCS− groups. (C) The Leicester cough questionnaire (LCQ) between MCS+ and MCS− groups. (D) The cough threshold for cough sensitivity related to chemical stimulation (LgC5 of capsaicin) between MCS+ and MCS− groups. (E) The percentage of mechanical stimulation-related cough hypersensitivity (MCS+) in patients with chronic cough before and after treatment. (F) Venn diagram of cough sensitivity related to mechanical and chemical stimulation. (G) Difference in cough sensitivity related to chemical stimulation between MCS+ and MCS− groups. (H) The percentage of MCS+ and Cap+ among different phenotypes of chronic cough. (I) LgC5 of capsaicin across chronic cough phenotypes. (J) Multivariate logistic regression analysis of the risk factors for gastroesophageal reflux-related cough (GERC). Abbreviations: C5, the lowest concentration of capsaicin inducing 5 coughs or more; CRC, corticosteroid-responsive cough; GERC, gastroesophageal reflux-related cough; LCQ, Leicester cough questionnaire; MCS, mechanical stimulation-related cough sensitivity; Post, post-treatment; Pre, pre-treatment; RCC, refractory chronic cough; TVT, tracheal vibration test; UACS, upper airway cough syndrome; UTC, urge to cough; VAS, visual analog scale. *p<0.05, **p<0.01.
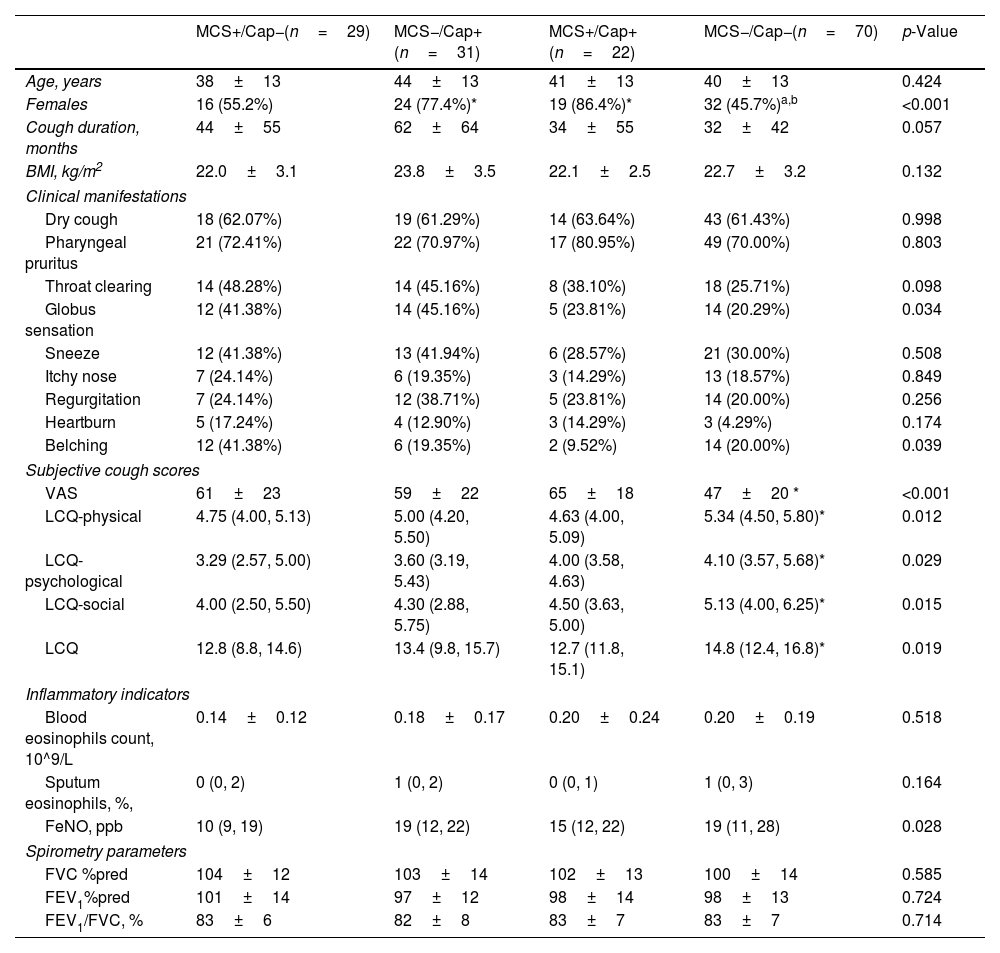
Among 152 patients, 14.4% were hypersensitive to both mechanical and chemical stimulation, 20.4% to chemical stimulation only, 19.1% to mechanical stimulation only, and 46.1% to neither (Fig. 1F). The Mosaic Plot showed a weak positive correlation between chronic cough patients sensitive to mechanical stimulation and capsaicin (Phi-coefficient=0.12, Fig. 1G). Females were predominant in the MCS−/Cap+ group (77.4%) and the MCS+/Cap+ group (86.4%), which was significantly higher than in the MCS+/Cap− group (55.2%) and the MCS−/Cap− group (45.7%, all p<0.001). Compared to the MCS−/Cap− group, the MCS+/Cap− group had higher VAS and lower LCQ scores (all p<0.01, Table 1).
Characteristics of Patients With Different Phenotypes of Cough Sensitivity.
| MCS+/Cap−(n=29) | MCS−/Cap+(n=31) | MCS+/Cap+(n=22) | MCS−/Cap−(n=70) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 38±13 | 44±13 | 41±13 | 40±13 | 0.424 |
| Females | 16 (55.2%) | 24 (77.4%)* | 19 (86.4%)* | 32 (45.7%)a,b | <0.001 |
| Cough duration, months | 44±55 | 62±64 | 34±55 | 32±42 | 0.057 |
| BMI, kg/m2 | 22.0±3.1 | 23.8±3.5 | 22.1±2.5 | 22.7±3.2 | 0.132 |
| Clinical manifestations | |||||
| Dry cough | 18 (62.07%) | 19 (61.29%) | 14 (63.64%) | 43 (61.43%) | 0.998 |
| Pharyngeal pruritus | 21 (72.41%) | 22 (70.97%) | 17 (80.95%) | 49 (70.00%) | 0.803 |
| Throat clearing | 14 (48.28%) | 14 (45.16%) | 8 (38.10%) | 18 (25.71%) | 0.098 |
| Globus sensation | 12 (41.38%) | 14 (45.16%) | 5 (23.81%) | 14 (20.29%) | 0.034 |
| Sneeze | 12 (41.38%) | 13 (41.94%) | 6 (28.57%) | 21 (30.00%) | 0.508 |
| Itchy nose | 7 (24.14%) | 6 (19.35%) | 3 (14.29%) | 13 (18.57%) | 0.849 |
| Regurgitation | 7 (24.14%) | 12 (38.71%) | 5 (23.81%) | 14 (20.00%) | 0.256 |
| Heartburn | 5 (17.24%) | 4 (12.90%) | 3 (14.29%) | 3 (4.29%) | 0.174 |
| Belching | 12 (41.38%) | 6 (19.35%) | 2 (9.52%) | 14 (20.00%) | 0.039 |
| Subjective cough scores | |||||
| VAS | 61±23 | 59±22 | 65±18 | 47±20 * | <0.001 |
| LCQ-physical | 4.75 (4.00, 5.13) | 5.00 (4.20, 5.50) | 4.63 (4.00, 5.09) | 5.34 (4.50, 5.80)* | 0.012 |
| LCQ-psychological | 3.29 (2.57, 5.00) | 3.60 (3.19, 5.43) | 4.00 (3.58, 4.63) | 4.10 (3.57, 5.68)* | 0.029 |
| LCQ-social | 4.00 (2.50, 5.50) | 4.30 (2.88, 5.75) | 4.50 (3.63, 5.00) | 5.13 (4.00, 6.25)* | 0.015 |
| LCQ | 12.8 (8.8, 14.6) | 13.4 (9.8, 15.7) | 12.7 (11.8, 15.1) | 14.8 (12.4, 16.8)* | 0.019 |
| Inflammatory indicators | |||||
| Blood eosinophils count, 10^9/L | 0.14±0.12 | 0.18±0.17 | 0.20±0.24 | 0.20±0.19 | 0.518 |
| Sputum eosinophils, %, | 0 (0, 2) | 1 (0, 2) | 0 (0, 1) | 1 (0, 3) | 0.164 |
| FeNO, ppb | 10 (9, 19) | 19 (12, 22) | 15 (12, 22) | 19 (11, 28) | 0.028 |
| Spirometry parameters | |||||
| FVC %pred | 104±12 | 103±14 | 102±13 | 100±14 | 0.585 |
| FEV1%pred | 101±14 | 97±12 | 98±14 | 98±13 | 0.724 |
| FEV1/FVC, % | 83±6 | 82±8 | 83±7 | 83±7 | 0.714 |
Data are presented as mean±SD, median (IQR) or n (%).
Abbreviations: MCS, mechanical stimulation-related cough sensitivity; Cap, chemical stimulation-related cough sensitivity; BMI, body mass index; VAS, visual analog scale; LCQ, Leicester cough questionnaire; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; CRC, corticosteroid-responsive cough; GERC, gastroesophageal reflux-related cough; RCC, refractory chronic cough; UACS, upper airway cough syndrome.
Cough and/or urge to cough induced by the tracheal vibration test (65Hz) within 30s were considered as mechanical stimulation-related cough hypersensitivity. Capsaicin cough hypersensitivity was defined as LgC5≤0.89μmol/L.
The incidence of MCS+ was 65.0% in GERC, 19.1% in corticosteroid-responsive cough, 27.3% in refractory chronic cough, and 25.9% in upper airway cough syndrome, respectively. Patients with GERC showed a higher incidence of MCS+ than those with corticosteroid-responsive cough, refractory chronic cough, and upper airway cough syndrome (all p<0.05) (Fig. 1H). No significant difference was observed in the incidence of LgC5 among groups with different clinical phenotypes (Fig. 1I). Logistic regression models identified three independent GERC predictors: belching (OR 3.24 [95% CI 1.30–8.08]; p=0.012), meal (OR 3.34 [95% CI 1.37–8.17]; p=0.008), MCS+ (OR 4.76 [95% CI 2.03–11.21]; p<0.001) (Fig. 1J, Appendix Table S3).
Cough hypersensitivity syndrome is defined as troublesome coughing often triggered by low levels of thermal, mechanical or chemical exposure.9 In our study, we found that about one-third of patients with chronic cough were sensitive to mechanical stimulation of the trachea. These patients had more severe subjective cough symptoms and the incidence of MCS+ decreases after treatment. Both mechanical and chemical stimulation can alter the activity of the efferent cough pathway or cough-related brain regions, leading to more severe symptoms, and both of them may be reversible features in chronic cough.10
This study is the first to demonstrate the heterogeneity of mechanical and chemical stimulation cough hypersensitivity in patients with chronic cough. The heterogeneity of cough sensitivity could be explained by anatomical differences in nerve pathways of cough reflex. An increased density of airway epithelial sensory nerves has been suggested as a pathological mechanism for cough hypersensitivity.11 However, Chung's study found that LgC5 of capsaicin was negatively correlated with the expression of TRPV1 but not PGP9.5.12 Considering that different cough receptors perceive different appropriate stimulation, it can be speculated that the type of airway epithelial neural remodeling differs for different types of cough hypersensitivity.13 In addition, cough-related vagal sensory fibers comprise myelinated Aδ fibers (mechanical sensing) and unmyelinated C fibers (chemical sensing), with their cell bodies predominantly localized to the nodose and jugular ganglia, respectively.14 In recent years, there have been studies on cough medicines targeting cough receptors.15 The P2X3 antagonist drug Gefapixant was effective in improving cough symptom, while it significantly improved ATP and distilled water cough sensitivity, but failed to improve capsaicin and citric acid cough sensitivity.16 This suggests that a more comprehensive assessment of cough sensitivity and individualized targeting of different cough hypersensitivity pathways may be warranted. Combined mechanical and chemical stimulation cough challenge tests can help to individualize the identification of cough hypersensitivity phenotypes and help explore the mechanisms of cough hypersensitivity.
Patients with GERC exhibited a significantly higher incidence of MCS+ compared to other phenotypes of chronic cough. Moreover, the presence of MCS+ was an independent risk factor for GERC. The potential pathological mechanism of the association between GERC and MCS remains unclear. Visceral sensory nerve inputs stimulated by tracheal vibration and pathological reflux or dysmotility in the esophagus were converge in the nodose ganglia.14 It was reported that active neuronal activity in the ascending pathway (including nodose ganglia) was involved in modulating airway neurogenic inflammation related to reflux.17,18 Lowenstein et al. identified Prox2 and Runx3 vagal sensory neurons in nodose ganglia functioning as mechanoreceptors and regulating esophageal motility.19 It can be hypothesized that sensitization of the shared mechanosensitive afferent neural pathways between the trachea and esophagus may underlie the heightened cough hypersensitivity to mechanical stimulation observed in patients with GERC. The value of the TVT in determining the GERC phenotype of chronic cough deserves further investigation.
Firstly, as mechanosensitive cough receptors were principally within the larynx, trachea, and main bronchus, the TVT can’t cover all the evoked points.20 Secondly, the reproducibility and positive criteria for TVT established in this cohort require external validation. Thirdly, the relationship between MCS and GERC remains to be further explored by prospective research with a larger sample size. Additionally, while different clinical phenotypes of chronic cough patients were included in this study, the treatment methods were not uniform, so the relationship between MCS and treatment effect needs targeted validation in the future. Finally, the single-center design needs further explore in multi-center cohorts to ensure generalizability.
In summary, there is heterogeneity between mechanical and chemical stimulation cough sensitivity. Chronic cough patients with MCS+ report more severe cough, and are more likely to be associated with reflux-induced cough. The integration of mechanical and chemical stimulation cough sensitivity helps identify a broader spectrum of cough hypersensitivity, which can aid in refining individualized management strategies for chronic cough.
Author ContributionsRC proposed the conception of the study. RC, ZY and CZ designed the study. RC, ZY, and CZ had full access to the data and gave final approval of the version to be published. LF, SZ, MZ, SZ, HZ and CZ involved in the statistical analysis and drafted the manuscript. LF, SZ, XS, MX, and WW recruited subjects. LF, ZF, BL, XJ, and WL collected data and had verified the underlying data. WL, JL, and KL revised the manuscript for important intellectual content. All authors revised the manuscript and approved the final version.
Data SharingThe data that support the findings of this study are available from the corresponding authors (RC, ZY, CZ) upon reasonable request and with permission of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Ethics StatementsThis study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (IRB: 2019-11).
Generative AIWe confirm that no content in this manuscript has been generated using AI tools.
FundingThis work was financially supported by Guangzhou National Laboratory (GZNL2024A02002), China International Medical Foundation (Z-2017-24-2301), National Natural Science Foundation of China (82170024, 82200030), Guangzhou Fundamental Research Programme (2024A03J1142), Guangdong Basic and Applied Basic Research Foundation (2022B1515120055), State Key Laboratory of Respiratory Diseases (SKLRD-Z-202327).
Conflict of InterestRuchong Chen reports a relationship with AstraZeneca, GSK, GENRIX BIO, Novartis, and Sanof that includes: funding grants and speaking and lecture fees. Jing Li reports a relationship with AstraZeneca, GSK, Novartis, and Sanof that includes: funding grants and speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors acknowledge participants participating in the study, and all of physicians and nurses who cared for these patients in the First Affiliated Hospital of Guangzhou Medical University.