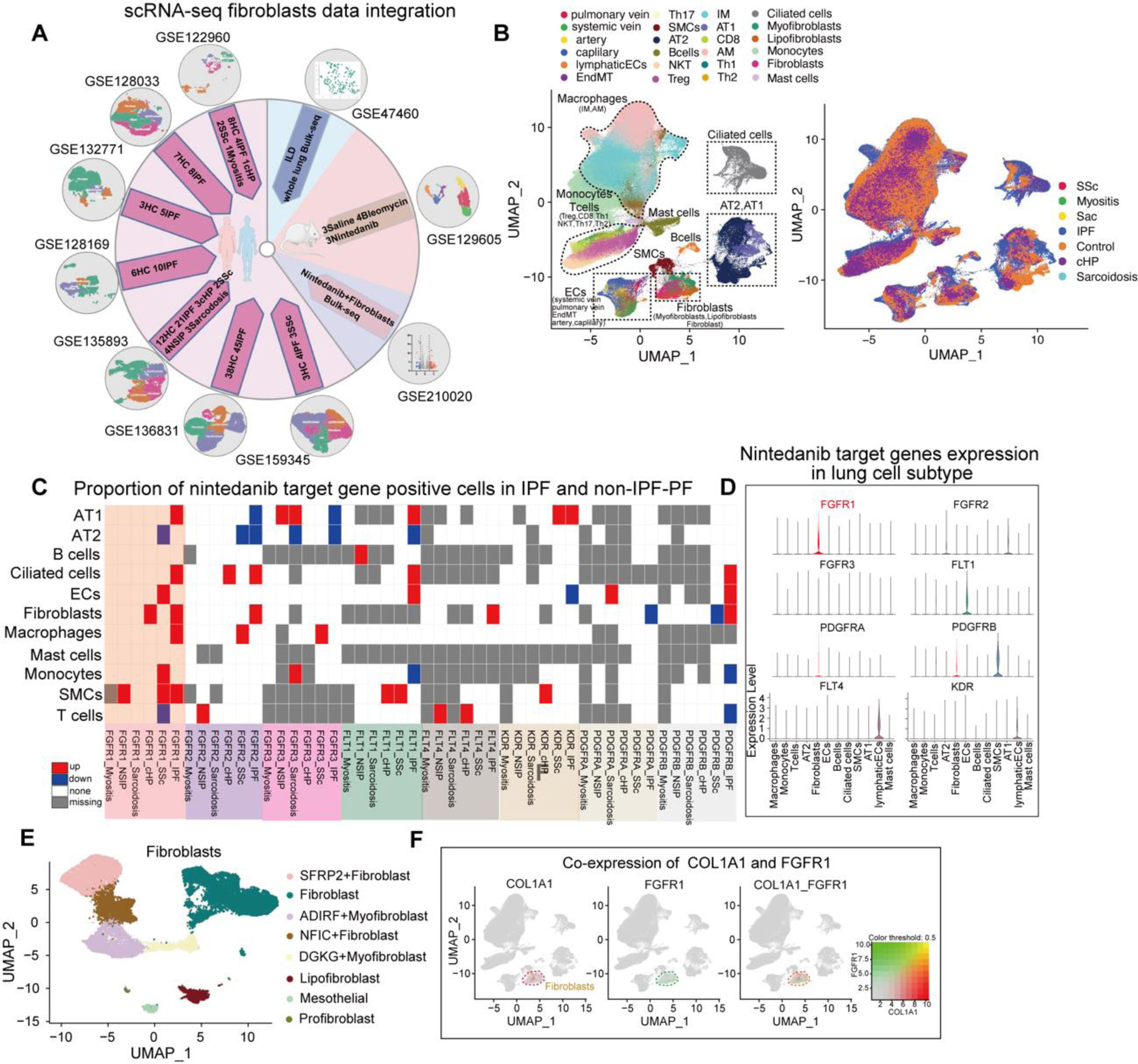
Idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF; e.g., systemic sclerosis-associated interstitial lung disease [SSc-ILD], hypersensitivity pneumonia [HP], and connective tissue disease-related ILD) exhibit overlapping fibrotic pathways, despite representing distinct clinical entities.1,2 Both are characterized by progressive lung function decline and poor prognoses, with current standard therapies proving ineffective in halting fibrosis. Antifibrotic agents such as nintedanib, approved for the management of IPF, offer a promising alternative.3 Nintedanib targets key kinases (such as the vascular endothelial growth factor (VEGF) receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR)) involved in fibrogenesis, slowing the decline in forced vital capacity in patients with IPF.4 Preclinical studies have shown that nintedanib has a remarkable and highly selective pharmacological activity, targeting all three VEGFR isoforms; FGFR1, 2, and 3; and kinases such as PDGFR-α and β.5–7 However, evidence of effectiveness of nintedanib remains limited beyond clinical trials on SSc-ILD.8,9 Ongoing trials aim to validate the broader application of nintedanib, leveraging its multitarget mechanism to address heterogeneous PPF subtypes. In this study, we aim to synthesize the expression profiles of nintedanib in IPF and PPF models, exploring its therapeutic potential across fibrotic ILDs. By bridging preclinical insights and emerging clinical data, the study seeks to establish a rationale for repurposing antifibrotics in PPF cases, ultimately guiding evidence-based strategies for the management of progressive fibrosis.
The inflammatory phase of the bleomycin-induced mouse model more closely resembles that of PPF, as evidenced by pathological features such as exacerbated inflammation and elevated mortality rates.10 Therefore, our study integrated seven human pulmonary fibrosis single-cell RNA-seq datasets, one single-cell dataset from a bleomycin-treated mouse model, a bulk-seq dataset of human pulmonary fibrosis (254 samples), and a bulk-seq dataset of nintedanib-treated human fibroblasts (Fig. 1A, sTable 1). The detailed protocols are provided in the Supplementary Material. We further merged fibroblasts from seven Gene Expression Omnibus (GEO) datasets, grouping cell subpopulations into endothelial cells (pulmonary vein, systemic vein, arteries, capillaries, and endothelial to mesenchymal transition), lymphatic endothelial cells, type I alveolar epithelial cells, type II alveolar epithelial cells, ciliated cells, monocytes, macrophages (interstitial macrophages and alveolar macrophages), T cells (CD8, Th1, Th2, Th17, Treg, and NKT), mast cells, B cells, fibroblasts (fibroblasts, myofibroblasts, and lipofibroblasts), and smooth muscle cells (Fig. 1B, sFig. 1A). We found significantly higher proportions of cells exhibiting positivity for 15 nintedanib target genes in all subpopulations derived from patients with IPF compared to those with other ILD types; further, FGFR1-positive cells showed the most prominent increase (Fig. 1C, sFig. 1B–D). Additionally, FGFR1 expression was elevated in the fibroblasts of patients with IPF and HP, and COL1A1 correlated with the distribution of FGFR1 in cell subpopulations (Fig. 1D, E). These findings suggest a significant elevation of FGFR1 expression in fibroblasts, particularly in patients with IPF and HP (sFig. 1E).
Identification of nintedanib-sensitive fibroblast subpopulations in IPF patients through combined scRNA-seq and Bulk-seq analysis. (A) Flow chart of the joint analysis of the human scRNA-seq and bulk_seq datasets and schematic diagram of the original publication of the dataset. (B) UMAP plots depicting the single-cell cluster analysis of lung tissue from ILD patients and healthy controls, identifying 24 distinct cell subpopulations: endothelial cells (pulmonary vein, systemic vein, arteries, capillaries, and endothelial to mesenchymal transition (EndMT)), lymphatic endothelial cells (lymphaticECs), type I alveolar epithelial cells (AT1), type II alveolar epithelial cells (AT2), ciliated cells, monocytes, macrophages (interstitial macrophages(IM) and alveolar macrophages(AM)), T cells (CD8, Th1, Th2, Th17, Treg, and NKT), mast cells, B cells, fibroblasts (fibroblasts, myofibroblasts, and lipofibroblasts), and smooth muscle cells (SMCs). In addition, UMAP plots grouped by disease classification are presented, including healthy controls, idiopathic pulmonary fibrosis (IPF), and other types of PPF-such as chronic hypersensitivity pneumonitis (HP), systemic sclerosis (SSc), myositis, non-specific interstitial pneumonia (NSIP), and sarcoidosis. (C) Heat map showing the results for each cell subpopulation (AT1, AT2, B cells, Ciliated cells, Endothelial cells, Fibroblasts, Macrophages, and myositis-ILD) in patients with different types of ILD (IPF, chronic hypersensitivity pneumonitis (HP), nonspecific interstitial pneumonia (NSIP), sarcoidosis, systemic sclerosis (SSs), myositis-ILD) versus healthy control lung tissue for each cell subpopulation (AT1, AT2, B cells, Ciliated cells, Endothelial cells, Fibroblasts, Macrophages, Mast cells, Monocytes, Smooth muscle cells, T cells) in a meta-analysis of the difference in the percentage of nintedanib target gene positive cells: “PDGFRB”, “PDGFRA”, “KDR”, “FLT4”, “FLT1”, “FGFR3”, “FGFR2”, “FGFR1”. (Red indicates upregulation, blue indicates downregulation, white indicates no significant change, and gray indicates no expression). (D) Violin diagram demonstrating the expression of eight nintedanib target genes in different cell subpopulations. Expression of FGFR1 was significantly elevated in fibroblasts. (E) UMAP plots of lung tissue fibroblasts from patients in the ILD group and healthy controls clustered into 8 cell subgroups. (F) Umap plot showing both the co-expression of the fibroblast marker gene COL1A1 and the nintedanib target gene FGFR1.
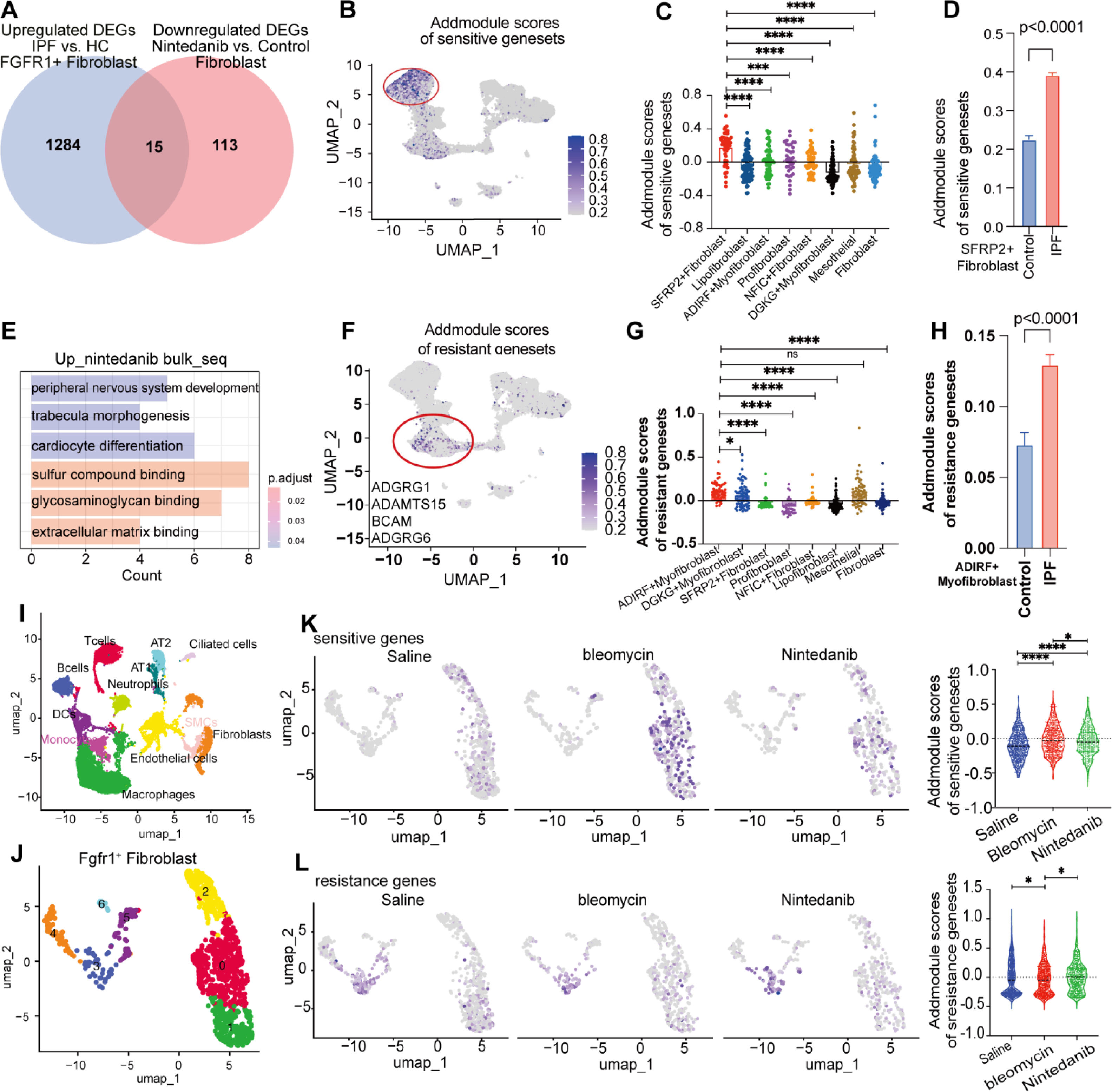
We integrated fibroblasts from seven GEO datasets. Further, we systematically identified pulmonary cell subpopulations by integrating data from the Human Lung Cell Atlas11 and employing well-established fibroblast markers reported in previously published seminal works.12–14 Subsequently, we classified the cell clusters into eight subgroups, as follows: profibroblasts, mesothelial cells, lipofibroblasts, DGKG+myofibroblasts, ADIRF+myofibroblasts, NFIC+fibroblasts, PCDHGA1+fibroblasts, and SFRP2+fibroblasts (sFig. 2A, B). Analysis of FGFR1-positive lung fibroblasts from patients with IPF revealed 1299 significantly upregulated genes (sFig. 2C). After nintedanib intervention, 128 genes were downregulated in fibroblasts, highlighting its impact (sFig. 2D). We identified 15 shared nintedanib-sensitive differentially expressed genes (DEGs) by comparing upregulated genes in IPF fibroblasts with downregulated genes after nintedanib treatment (Fig. 2A), providing insights for targeted therapies in fibrotic lung diseases.
Identification of nintedanib-sensitive fibroblast subpopulations in IPF patients through combined scRNA-seq and bulk RNA-seq analysis. (A) Venn diagram showing the overlap of upregulated differentially expressed genes (DEGs) in FGFR1+ fibroblasts (IPF vs. healthy controls) and downregulated genes following nintedanib treatment in fibroblasts. A total of 15 genes were identified: ARF4, PHLDA1, TMED2, RPN1, TNFAIP6, PRDX6, C5orf15, EGR1, TPBG, CALR, KCTD12, SSR1, FKBP14, DUSP6, and THBS1. (B–D) UMAP visualization of fibroblast subpopulations showing that the SFRP2+ fibroblast subset is significantly enriched for the nintedanib-sensitive gene set in IPF patients compared to healthy controls. Histogram plots illustrate that SFRP2+ fibroblasts have significantly higher AddModule scores compared to other fibroblast subsets. Additionally, IPF patients exhibited a significantly greater proportion of SFRP2+ fibroblasts with higher sensitive gene set scores than healthy controls. (E) GO enrichment analysis of upregulated genes following nintedanib treatment in fibroblasts, highlighting significant enrichment in fibrosis-related pathways, including extracellular matrix binding. (F–H) UMAP visualization of fibroblast subpopulations showing that the ADIRF+ myofibroblast subset is significantly enriched for the nintedanib-resistance gene set in IPF patients compared to healthy controls. Histogram plots reveal that ADIRF+ myofibroblasts have significantly higher AddModule scores relative to other fibroblast subsets. Moreover, patients with IPF showed significant enrichment of the drug-resistance gene set in the ADIRF+ myofibroblast population compared to healthy controls. (I) UMAP plots of single-cell cluster analysis of lung tissues from bleomycin-treated mice, nintedanib-treated mice, and saline-treated controls, identifying 11 major cell subpopulations from a total of 18,697 cells. (J) UMAP plot of FGFR1+ positive fibroblasts. (K) AddModule scoring of the nintedanib-sensitive gene set in venous endothelial cells revealed that the fibrosis score was significantly elevated in the bleomycin group compared to the saline group, and was reduced following nintedanib treatment. (L) AddModule scoring of the nintedanib-resistance gene set in venous endothelial cells showed that fibrosis scores were significantly higher in the bleomycin group compared to the saline group, and further increased after nintedanib treatment.
Further analysis of these 15 DEGs in IPF and PPF cases (sFig. 2E) revealed a nintedanib-sensitive fibroblast subpopulation (SFRP2+fibroblast), which was significantly more prominent in patients with IPF than in controls (Fig. 2B–D). Bulk-seq analysis of ILD cases identified seven genes that were negatively correlated with lung function (sFig. 2F). In patients with HP, 30 nintedanib-sensitive genes were highly expressed in the SFRP2+fibroblast subpopulation and elevated in patients with HP compared with that in controls (sFig. 2G–J). We also identified 11 genes in lung bulk-seq data that were negatively associated with lung function (sFig. 2J–K). Gene Expression Profiling Interactive Analysis (GEPIA) analysis of The Cancer Genome Atlas Program (TCGA) lung adenocarcinoma data revealed that high TPBG expression, found in both HP and IPF fibroblasts, predicts low survival rates (sFig. 2L). Additionally, TPBG expression correlated with mutant gene expression in lung adenocarcinoma, including that of EGFR, BRCA, TP53, and KRAS (sFig. 2M).
Furthermore, we aimed to identify the gene set associated with resistance to nintedanib. We analyzed DEGs upregulated in fibroblasts after nintedanib treatment and found them to be significantly enriched in fibronectin-related signaling pathways (Fig. 2E). Clustering analysis identified a subpopulation of ADIRF myofibroblasts resistant to nintedanib (Fig. 2F, G). This subpopulation showed significantly higher scores in patients with IPF compared with that in controls (Fig. 2H). Our investigation of drug resistance genes revealed that ADGRG1 was negatively associated with lung function (sFig. 2N). GEPIA analysis of TCGA lung adenocarcinoma data showed that high ADGRG1 expression did not correlate with survival (sFig. 2O). We further examined human lung data from nintedanib-treated bleomycin model mice, identifying 11 major cell subpopulations and 18,697 cells (Fig. 2I, sFig. 3A–C). After extracting FGFR1-positive fibroblasts (Fig. 2J), we found that the nintedanib-sensitive gene cluster had significantly higher scores in the bleomycin group than in the control group, and the scores decreased after nintedanib treatment (Fig. 2K). Additionally, the gene set for drug resistance increased significantly after nintedanib treatment (Fig. 2L). Identifying gene signatures within nintedanib-resistant fibroblast subpopulations helps in selecting patients for nintedanib therapy.
Recent scRNA-seq advancements have elucidated cellular heterogeneity and drug mechanisms in pulmonary fibrosis. As a common target subpopulation of nintedanib, fibroblasts were analyzed in this study. Additionally, we confirmed that FGFR1 exhibits the highest expression level in fibroblasts. We identified nintedanib-sensitive cell subpopulations, and the associated genes were found to be correlated with lung function. We identified nintedanib-sensitive and -resistant fibroblast subpopulations in IPF and PPF cases, correlating with lung function decline.
HP was classified into nonfibrotic and fibrotic phenotypes in 2021 by the American Thoracic Society, Japanese Respiratory Society, and Asociación Latinoamericana del Tórax guidelines.15–17 Existing studies show that nintedanib demonstrates optimal efficacy in IPF and SSc by targeting kinases (such as FGFR1-3, PDGFRα, and VEGFRs), aligning with Phase III trial data. However, its efficacy in HP cases remains unproven.9 Trials such as INBUILD support its use in progressive fibrosis, while observational studies report stabilized lung function in patients with HP18 Pirfenidone, another antifibrotic, showed mixed results in HP trials, underscoring the need for tailored therapies. Our findings demonstrate that while nintedanib is primarily indicated for IPF, it also exhibits potential therapeutic efficacy in hypersensitivity pneumonitis.
Intriguingly, nintedanib-sensitive fibroblast subpopulations were associated with pulmonary function and lung adenocarcinoma mutations (TCGA data), suggesting their cross-disease therapeutic potential.19 Studies indicate that nintedanib exerts anti-tumor effects by modulating CD8+ T cells and suppressing fibroblasts, highlighting its dual role in fibrosis and cancer.20 Therefore, we screened and identified the gene TPBG, whose upregulated expression is inhibited by nintedanib. Research demonstrates that TPBG plays a critical role in tumor progression. In fibroblasts, TPBG silencing reduced pro-fibrotic CXCL12/CXCR7 signaling and angiogenesis, while TPBG overexpression enhanced these pathways, mediated by the CXCR7/CXCR4 heterodimer, thereby influencing tumorigenesis.
In summary, our findings highlight nintedanib as a potential therapeutic target for HP. Furthermore, we have for the first time identified therapy-sensitive and -resistant gene signatures in PPF, further confirming the role of nintedanib in adjunctive cancer therapy. These valuable insights provide critical information on the therapeutic potential of nintedanib for both pulmonary diseases and tumor.
Authors’ ContributionsLZ conceived the topic for this study. XZ and XT performed scRNA and bulk RNA sequencing. CL conducted data analysis. LZ, XZ, CL, YS, and XF contributed to the writing and revising of the manuscript. LZ, XZ, XF, and CL critically revised the manuscript. All authors listed have read and approved the manuscript before submission.
Ethics Approval and Consent to ParticipateNot applicable.
Consent for PublicationAll authors consented for publication.
FundingThe study was supported by the Technology Cooperation Project of Chengdu (2023-GH02-00092-HZ), the Youth Innovation Project of Sichuan Medical Association (Q21018), Chengdu Medical Research Projects (2022516).
Conflict of InterestsThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Availability of Data and MaterialThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The authors thank Dr. Jianming Zeng (University of Macau), and all the members of his bioinformatics team, biotrainee, for generously sharing their experience and codes.