Alpha-1 antitrypsin deficiency (AATD) is a rare hereditary condition derived from mutations in the SERPINA1 gene that encodes the alpha-1 antitrypsin (AAT) protein. It is a complex monogenic disorder since, based on the same genetic mutation, it presents great variability in clinical manifestations. In this way, the disease predisposes to the appearance of certain respiratory conditions, such as pulmonary emphysema; liver diseases, such as liver cirrhosis and hepatocellular carcinoma; and, less frequently, other diseases, such as neutrophilic panniculitis or ANCA+systemic vasculitis. The most common mutations related to this condition are Pi*Z and Pi*S, with Pi*M being the variant that encodes the functional protein.1,2
Neutrophils have been shown to play a key role in AATD,3,4 which is also considered an inflammatory disorder. Although these cells are essential for the host defense, activated neutrophils can also lead to tissue damage through the release of several proteases and reactive oxygen species (ROS). Several observations have highlighted the neutrophils’ role in AATD pathophysiology. First, the burden of neutrophils is increased in the lungs of AATD patients compared to healthy volunteers.5,6 Second, an increased number of neutrophils was observed in AATD patients, which correlates with decreased lung function.4
On the other hand, in processes such as infection or inflammation, the affected tissue becomes profoundly hypoxic. Neutrophils have evolved to function effectively under these conditions since local hypoxia is part of the normal inflammatory process. Furthermore, neutrophil survival is shown to be increased under hypoxic conditions, which contributes to the delay in the resolution of inflammation and increases tissue damage.7 In addition, several studies have highlighted the fact that neutrophils play an important role in the pathophysiology of several respiratory diseases associated with tissue hypoxia, such as neutrophilic asthma,8 chronic obstructive pulmonary disease (COPD),9 bronchiectasis,8 cystic fibrosis10 and AATD.11,12
Therefore, the present study aimed to investigate the behavior of AATD neutrophils under hypoxic conditions. AAT levels secreted by neutrophils isolated from children and adult patients with AATD were compared under normoxic (21% O2) vs. hypoxic (1% O2) conditions.
Regarding the study in the pediatric population, fifty-four AATD children (31 MZ; 8 SZ; 15 ZZ) and seven healthy volunteers were recruited from the Pediatrics service of Hospital Clínico Universitario de Valencia (HCUV) and Hospital de Manises (Valencia). Fourteen adult AATD patients (9 MZ; 6 ZZ) and 15 healthy volunteers were recruited from the Pulmonology services of HCUV and Hospital Universitario Doctor Peset (Valencia). Research ethics committees of the participating hospitals approved the study (see Institutional Review Board Statement in supplementary file). AATD patients were grouped according to their phenotype (MZ, SZ, and ZZ). The serum concentration of AAT was measured by nephelometry. AAT phenotypes were determined by isoelectric focusing of serum samples, as previously described,15 and differences were reported according to the individuals’ phenotypes.
Online Supplementary Tables S1 and S2 display the clinical and demographic details of the study's patients. Subjects included in the study showed no statistically significant differences in age, sex, or body mass index. All subjects included in this study were clinically healthy according to both their physical status and their pulmonary and liver tests. No significant differences were observed in hematological inflammatory markers between the groups, indicating that systemic inflammation is not a differential factor in AATD.
Peripheral venous blood was collected in Vacutainer tubes containing K2EDTA (Becton Dickinson, Cat. #367856) from AATD patients and healthy volunteers. Neutrophils were purified by negative immunomagnetic selection (EasySep™, StemCell Technologies, Cat. #19666) according to the manufacturer's instructions. Purified cells were incubated at 9×105cells/mL in RPMI (Sigma–Aldrich, Cat. #R8758) supplemented with 10% inactivated fetal bovine serum (Sigma–Aldrich, Cat. #F7524), 1% sodium pyruvate (Sigma–Aldrich, Cat. #S8636), and 1% non-essential amino acids (Sigma–Aldrich, Cat. #M7145) under normoxia (21% O2) or hypoxia (1% O2) for 4h at 37°C. Neutrophil priming was performed with tumor necrosis factor-alpha (TNFα) (20ng/mL) for 30min, followed by the formylated peptide, N-Formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) (100nM) for 10min as previously described.9 Once the incubations were completed, samples were centrifuged at 2000×g at 4°C for 10min, and the supernatant harvested, aliquoted, and stored at −80°C until processed. The measurement of AAT in the supernatants was conducted using a commercial ELISA (Alpha-1 Antitrypsin (SERPINA1) Human in vitro ELISA; Abcam, Cat. #ab108799), according to the manufacturer's instructions.
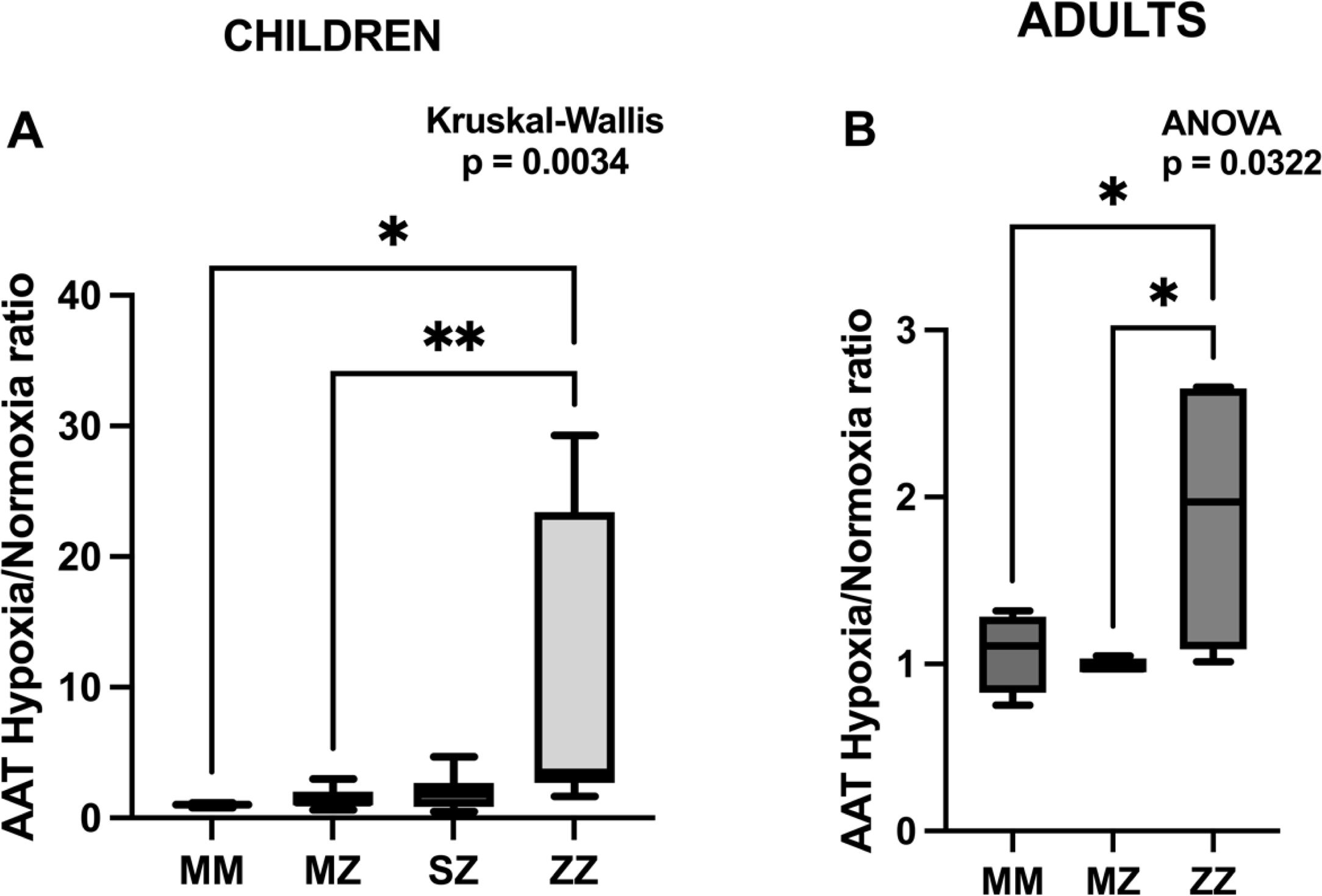
As mentioned above, this study aims to identify differences in the AAT released by neutrophils isolated from AATD patients cultured under hypoxic conditions to evaluate possible phenotype and age-associated differences. The results are expressed as the hypoxia/normoxia ratio (Fig. 1A and B). This approach has been chosen instead of presenting the absolute values of hypoxia and normoxia separately (Supplementary Fig. S1) to normalize individual variability, allowing a better comparison between different phenotypes. This strategy enhances the relative changes in AAT levels produced by hypoxia, as opposed to the absolute values, which are more influenced by other factors such as genetic, clinicopathological, and environmental factors. Also, using the ratio gives the study greater statistical power as individual variability is reduced.
AAT expression under hypoxic conditions in different genotypes. Increased AAT production under hypoxia relative to normoxia in ZZ individuals, suggesting a genotype-specific response, is observed. (A) Box plot showing the ratio of alpha-1 antitrypsin (AAT) expression under hypoxia relative to normoxia for MM, MZ, SZ, and ZZ genotypes in children. Kruskal–Wallis test indicates significant differences between groups (*p<0.05, **p<0.01). (B) Comparison of AAT hypoxia/normoxia ratios between MM, MZ, and ZZ genotypes using ANOVA in adults. Statistically significant differences between them were observed (*p<0.05).
Fig. 1A (children) and B (adults) show box plots comparing the different phenotypes’ AAT hypoxia/normoxia ratios. In both age groups, a statistically significant difference was observed among the phenotypes (p=0.0034 in children and p=0.0322 in adults). In children, the MM, MZ, and SZ phenotypes showed relatively low and similar AAT ratios, whereas the ZZ phenotype showed a higher hypoxia/normoxia ratio. Statistical analysis showed statistically significant differences between the ZZ and the MM and MZ groups (p=0.028 and p=0.006), respectively), and no significant trend was observed with the SZ group (p=0.1584). Similarly, no significant differences were observed between the MM, MZ, and SZ groups.
Similar results were observed in adults, where the ZZ group also displayed the highest median value. Significant differences were observed between the ZZ and the MM and MZ groups (p=0.049 and p=0.0481, respectively). The overall AAT ratio in adults is lower than in children, suggesting a possible age-related decline in response to hypoxia.
Hypoxia plays a key role in inflammation, tissue remodeling, and disease progression in lung-related diseases.8,13 Since AATD neutrophils have been involved in the pathophysiology of the disease, studying the behavior of neutrophils under hypoxic conditions has physiological relevance. This study evaluated AAT production in neutrophils from AATD patients under normoxic (21% O2) and hypoxic (1% O2) conditions to assess hypoxia's role in AAT production. The study was performed on children and adults to evaluate possible age-associated differences. Our results indicate that the ZZ phenotype is consistently associated with an elevated AAT ratio under hypoxic conditions, regardless of age. However, the reduced AAT ratio in adults suggests that age may influence the regulation of AAT in response to hypoxia.
AAT is an acute phase reactant, and its expression is mainly induced by products of nitro-oxidative stress and inflammatory and infectious processes, such as bacterial endotoxin or lipopolysaccharide, interferon β or the cytokines interleukin-1, interleukin-6 and TNF alpha.6 Similarly, transcription activation of the SERPINA1 gene has been observed through mechanisms related to the protease–antiprotease balance, for example, by serin proteases, AAT-elastase complexes, or short peptides released from AAT-elastase complexes after being hydrolyzed.7 On the other hand, it has been shown that AATD individuals present high levels of pro-inflammatory cytokines in the lung epithelial lining fluid compared to healthy controls.14 ZZ-AATD neutrophils produce higher amounts of hydrogen peroxide, peroxynitrite, and nitric oxide than MM volunteers in hypoxic conditions.9 Increased levels of elastase have also been found in ZZ-AATD patients.14 At the systemic level, it has been observed that hypoxia induces the release of pro-inflammatory cytokines.15,16 Therefore, this increase in proteases, ROS, and pro-inflammatory cytokines in hypoxic ZZ lungs could explain the rise in AAT expression observed in ZZ patients under hypoxic conditions. In addition, previous studies by our research group have shown an increased ROS production, neutrophil degranulation, and inflammatory phenotype in AATD neutrophils under hypoxic conditions.17,18
It has previously been reported that hypoxia upregulates azurophil granule neutrophil release in vitro. Neutrophils isolated from healthy volunteers stimulated with GM-SCF and fMLP incubated 4h under hypoxia (3kPa) showed increased neutrophil elastase and myeloperoxidase release compared with those incubated under normoxia.9 Therefore, considering that azurophil granule also contains AAT, that observation agrees with our results.
This study has limitations that should be taken into account. First, the low number of patients included in the study, particularly in adulthood, is explained by the fact that this rare disease has a high degree of underdiagnosis. Second, and because of the above, we have not been able to explore the effects of possible medications taken by the patients, so their impact on the results could not be studied.
In summary, our findings show a potential phenotype-dependent regulation of AAT under hypoxic conditions. Neutrophils from ZZ individuals (both in adulthood and in pediatric age) exhibit distinct responses to hypoxia, with an elevated AAT ratio compared to the MM, MZ, and SZ groups), potentially linked to altered AAT regulation under low-oxygen conditions. Further studies are needed to elucidate the molecular mechanisms underlying this phenotype and age-dependent variation and its potential clinical implications.
Author contributionsConceptualization, FD, MM, SCC, AH, TR, and CG; methodology, MM, LB, DP; formal analysis, FD, MM; resources, SCC, AH, MFC, TR, CG; data curation, MM; writing—original draft preparation, FD, MM; writing—review and editing, MMNG; supervision, FD; project administration, FD, MMNG; funding acquisition, FD, MMNG, SCC. All authors have read and agreed to the published version of the manuscript.
Institutional review board statementThe Hospital Clínico Universitario de Valencia, Hospital Dr. Peset de Valencia, and Hospital de Manises (Valencia, Spain) Research Ethics Committees approved the study. Procedures were explained to the children and parents, and written informed consent was obtained from parents. The study was conducted according to the Ethical Principles for Medical Research on Human Beings of the Declaration of Helsinki.
Artificial intelligence involvementNo generative AI has been used to partially or totally produce any content of the article.
FundingThis study was funded by Sociedad Valenciana de Neumología (2017), ISCIII #PI17/01250 grants, and European Regional Development Funds (FEDER). María Magallón was funded by 2021 Amadeu Monteiro grant. Lucía Bañuls was supported by GVA grant number ACIF/2019/231.
Conflicts of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
We thank the Spanish association of patients with alpha-1 antitrypsin deficiency for donating funds to our research group on rare respiratory diseases at IIS INCLIVA/University of Valencia.