Both systemic inflammation and exacerbations have been associated with greater severity of bronchiectasis. Our objective was to analyze the prognostic value of the peripheral concentration of C-reactive protein (CRP) for the number and severity of exacerbations in patients with bronchiectasis.
MethodsPatients from the Spanish Bronchiectasis Registry (RIBRON) with valid data on their CRP value (in a clinically stable phase) and valid data on exacerbations during the first year of follow-up were included. A logistic regression analysis was used to evaluate the prognostic value of the CRP concentration (divided into tertiles) with the presence of at least one severe exacerbation or at least two mild-moderate exacerbations during the first year of follow-up.
Results802 patients (mean age: 68.1 [11.1 years], 65% female) were included. Of these, 33.8% and 13%, respectively, presented ≥2 mild-moderate exacerbations or at least one severe exacerbation during the first year of follow-up. The mean value of the CRP was 6.5 (17.6mg/L). Patients with a CRP value between 0.4 and 2.7mg/L (second tertile) and ≥2.7mg/L (third tertile) presented a 2.9 (95%CI: 1.4–5.9) and 4.2 (95%CI: 2.2–8.2) times greater probability, respectively, of experiencing a severe exacerbation than those with <0.4mg/L (control group), regardless of bronchiectasis severity or a history of previous exacerbations. However, the CRP value did not present any prognostic value for the number of mild-moderate exacerbations.
ConclusionsThe CRP value was associated with a greater risk of future severe exacerbations but not with mild or moderate exacerbations in patients with steady-state bronchiectasis.
Tanto la inflamación sistémica como las exacerbaciones se han asociado con una mayor gravedad de las bronquiectasias. Nuestro objetivo fue analizar el valor de la concentración en sangre periférica de proteína C reactiva (PCR) para predecir el número y la gravedad de las exacerbaciones en pacientes con bronquiectasias.
MétodosSe incluyeron pacientes del Registro Español de Pacientes con Bronquiectasias (RIBRON) con datos válidos sobre sus niveles de PCR (en fase clínicamente estable) y datos válidos sobre exacerbaciones durante el primer año de seguimiento. Se utilizó un análisis de regresión logística para evaluar el valor pronóstico de la concentración de PCR (dividida en terciles) con la presencia de al menos una exacerbación grave o al menos dos exacerbaciones leves-moderadas durante el primer año de seguimiento.
ResultadosSe incluyeron 802 pacientes (edad media: 68,1 [11,1] años, 65% mujeres). De ellos, el 33,8% y el 13%, respectivamente, presentaron ≥2 exacerbaciones leves-moderadas o al menos una exacerbación grave durante el primer año de seguimiento. El valor medio de la PCR fue de 6,5 (17,6) mg/L. Los pacientes con un valor de PCR entre 0,4 y 2,7mg/L (segundo tercil) y ≥2,7mg/L (tercer tercil) presentaron 2,9 veces (IC 95%: 1,4-5,9) y 4,2 veces (IC 95%: 2,2-8,2) más probabilidad, respectivamente, de experimentar una exacerbación grave que aquellos con <0,4mg/L (grupo de control), independientemente de la gravedad de las bronquiectasias o de presentar antecedentes de exacerbaciones previas. Sin embargo, el valor de la PCR no presentó ninguna utilidad pronóstica para el número de exacerbaciones leves-moderadas.
ConclusionesEl valor de la PCR se asoció a un mayor riesgo de exacerbaciones graves en el futuro, pero no a las exacerbaciones leves o moderadas en pacientes con bronquiectasias en fase estable.
Bronchiectasis (BE) is the third most prevalent chronic inflammatory airway disease, after asthma and chronic obstructive pulmonary disease (COPD), and it is closely related to both.1–4 It is usually presented as a clinical syndrome characterized by coughing, daily expectoration, and recurring episodes (exacerbations) of an infectious profile5–7 In February 2015, the Spanish Registry of Bronchiectasis (RIBRON)8 began to collect information and now has a well-characterized series of more than 2000 patients that makes it possible to conduct large-scale studies.
Both the number and severity of the exacerbations in BE patients have been shown to have a negative impact on the disease's natural history.9–15 In fact, some authors have mentioned the possible existence of a group of patients who share similar clinical pictures, prognoses, and treatments as a result of a particularly high number of exacerbations (“exacerbator phenotype”).16,17
Furthermore, a number of BE patients present an increase in some markers of systemic inflammation, which has been associated with greater severity of the disease.18–20 One of the inflammatory biomarkers most extensively studied is C-reactive protein (CRP).21 An increase in the systemic concentration of this molecule has been associated with greater severity of asthma, COPD, and cystic fibrosis (CF).22–27 However, data on the relationship between the concentration of CRP in clinically stable state and BE is still scarce, particularly with respect to the number and severity of exacerbations.28 Our working hypothesis is based on the fact that, as in the case of other inflammatory airway diseases, the peripheral concentration of CRP could be related to a greater number and severity of future exacerbations of BE. Thus, its evaluation in a stable phase of the disease may have a clinical application in such patients.
The objective of the present study is therefore to analyze the prognostic capacity of the peripheral levels of ultra-sensitive CRP (henceforth, CRP) on the frequency and severity of the exacerbations in a large series of BE patients taken from the RIBRON registry when they were in a clinically stable state.
MethodsStudy DesignProspective, observational, multicenter study of a cohort derived from the Spanish BE registry (RIBRON). This registry started in February 2015 and involves 43 centers from Spain. It includes adult patients diagnosed with BE by means of high-resolution computerized tomography (HRCT), with annual data on all of them and a monthly control of the quality of the data, undertaken by an outside company, to avoid any missing data. The cut-off point selected for the analyses of the present study was February 2019.
PatientsAt the time of the analysis data was available on 2039 patients. The criteria for inclusion were patients with BE with data available from at least one year of follow-up and at least one baseline measurement of the peripheral levels of CRP in conditions of clinical stability. Clinical stability was defined as at least 4 weeks free of an exacerbation period. The main criterion for exclusion was a diagnosis of CF.
All the patients included in the present investigation were informed about the purpose of the registry and any potential derived studies, and they gave their written informed consent in their corresponding participating center, as provided by the local ethics committee affiliated with the registry (number:001-2012. Hospital Josep Trueta. Girona).
VariablesThe RIBRON registry covers a wide range of both cross-sectional and longitudinal variables. The following were used for the purposes of this study: general and anthropometric data; etiology; scales of severity; lung function; treatments; number and severity of exacerbations; and analytical, radiological, and microbiological data.
To be included in the registry, an exacerbation was defined as a worsening of the typical symptoms of BE: cough; dyspnea; hemoptysis; increase in the volume or purulence of the sputum; chest pain; or sibilance with an evolution of more than 24h for which antibiotic treatment was required. In terms of severity, we classified the exacerbations into two subgroups: mild-moderate, if the patients needed oral antibiotics; and severe, in cases where hospital admittance or intravenous antibiotic treatment was required, even if the latter was administered at home (home hospitalization). Chronic bronchial infection (CBI) was defined as the presence of Three or more consecutive cultures positive for the same PPM.
Measurement of the Plasmatic Concentration of Ultrasensitive CRPCRP levels were determined in serum samples from all the study patients using immunoturbidimetry. Briefly, CRP levels were detected with the CRPL3 kit (Roche Diagnostics, Indianapolis, IN, USA) in a COBAS® 8000 modular analyzer (Roche Diagnostics), in which the samples were processed automatically. A standard curve was generated with each assay run. Intra and inter-assay coefficients of variation ranged from 0.6% to 3.7% in all cases. The minimum detectable concentration of serum CRP was set to be less than 0.2mg/L. CRP concentration was measured when patients were included in the registry for the baseline analysis in a clinically stable phase.
Statistical AnalysisThe quantitative variables were tabulated in terms of their mean (standard deviation) or median (interquartile range) according to their distribution. Qualitative variables were evaluated according to their relative frequency in percentage. The distribution of the variables was evaluated according to the Kolgomorov–Smirnov test. A multivariate logistic regression analysis was used to determine the independent prognostic value of the concentration of CRP with the greatest risk of an exacerbation (whether mild-moderate or severe) during the first year of follow-up. The variables used as confounders in the model were chosen by the authors because, according to the existing literature, they were thought the ones that could have a clinical impact on this relationship: age; gender; presence of CBI by Pseudomonas aeruginosa (PA); presence of CBI by other PPM; previous exacerbations; FEV1 in percentage of predicted value; the body mass index (BMI); the number of lobes affected; the presence of COPD as a comorbidity; the Charlson Index; the administration of inhaled corticosteroids (ICS), macrolides or inhaled antibiotics; and the degree of dyspnea according to the MRC scale. Furthermore, the three multidimensional scales of severity and prognosis of BE – the BSI (Bronchiectasis severity Index),29 E-FACED,30 and FACED31 – were also considered in a separate logistic regression analysis. In order to make the results easier for the reader to interpret, CRP was analyzed in tertiles. Moreover, a tree of the probability of severe exacerbation in the first year of follow-up was constructed, reflecting the presence or otherwise of variables where a greater prognostic value was observed, in the opinion of the authors, in either clinical or statistical terms. Risk was quantified according to the OR and the CI95%. In all cases, a P<.05 was considered significant. All the analyses were carried out by means of the SPSS package, version 21 (USA, Chicago).
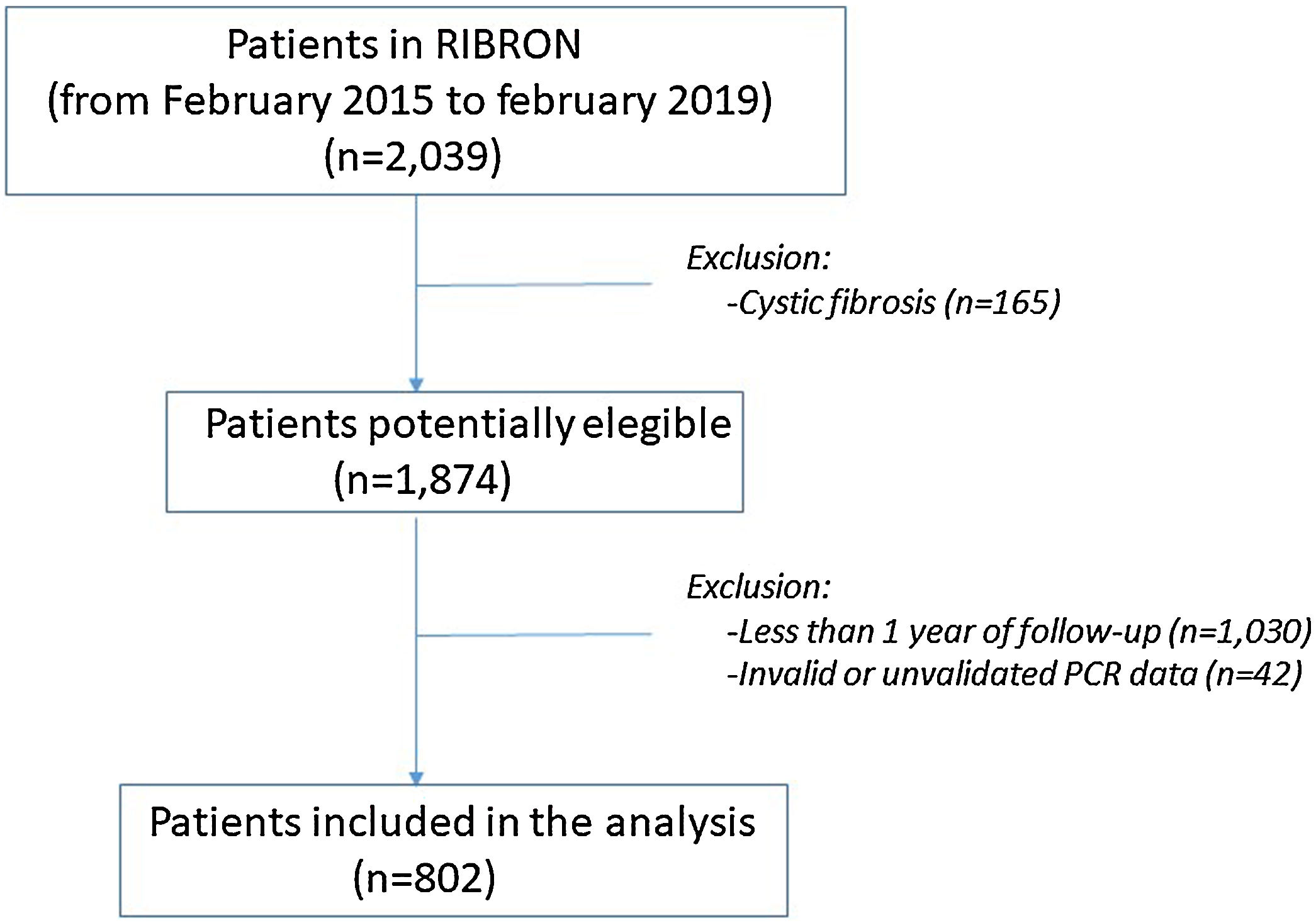
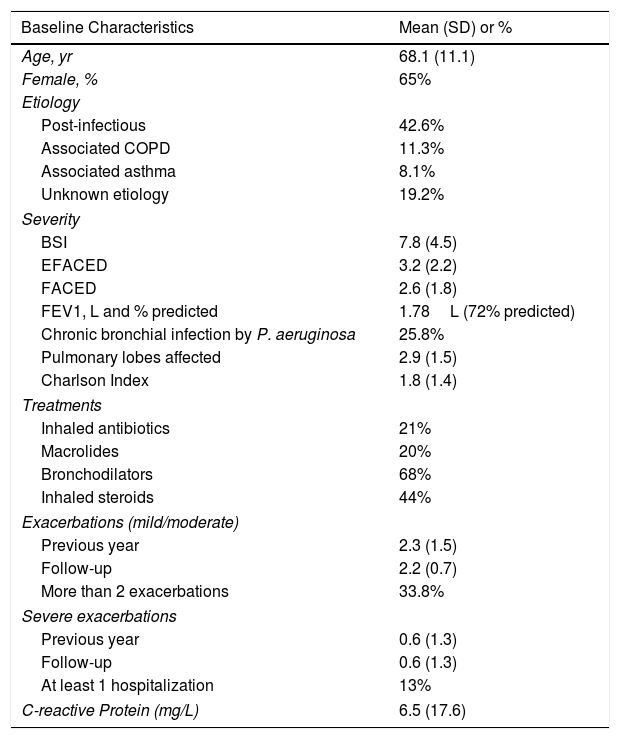
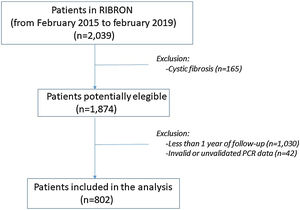
ResultsPatient characteristics at baseline are shown in Table 1. Of the 2039 patients included in the RIBRON at the time of the analysis, 802 (39.3%) were valid for the purposes of the study (Fig. 1). The patients who were not included presented CF (n=165), or they had not yet completed a year of follow-up in the registry (n=1030), or they presented invalid or non-validated CRP values (n=42). A comparison of these patients who were not included in the study with those who were did not reveal any significant differences (apart from the cases with CF) (data not shown). The baseline characteristics of the 802 patients included in the study are shown in Table 1.
Baseline Values of the Patients Finally Included in the Study.
| Baseline Characteristics | Mean (SD) or % |
|---|---|
| Age, yr | 68.1 (11.1) |
| Female, % | 65% |
| Etiology | |
| Post-infectious | 42.6% |
| Associated COPD | 11.3% |
| Associated asthma | 8.1% |
| Unknown etiology | 19.2% |
| Severity | |
| BSI | 7.8 (4.5) |
| EFACED | 3.2 (2.2) |
| FACED | 2.6 (1.8) |
| FEV1, L and % predicted | 1.78L (72% predicted) |
| Chronic bronchial infection by P. aeruginosa | 25.8% |
| Pulmonary lobes affected | 2.9 (1.5) |
| Charlson Index | 1.8 (1.4) |
| Treatments | |
| Inhaled antibiotics | 21% |
| Macrolides | 20% |
| Bronchodilators | 68% |
| Inhaled steroids | 44% |
| Exacerbations (mild/moderate) | |
| Previous year | 2.3 (1.5) |
| Follow-up | 2.2 (0.7) |
| More than 2 exacerbations | 33.8% |
| Severe exacerbations | |
| Previous year | 0.6 (1.3) |
| Follow-up | 0.6 (1.3) |
| At least 1 hospitalization | 13% |
| C-reactive Protein (mg/L) | 6.5 (17.6) |
The mean age was 68.1 (11.1) and 65% were female. The most common etiology was post-infection (42.6%). CBI by PA was found in 25.8% of the patients. The mean value (SD) of the CRP concentration was 6.5mg/L (17.6). 33.8% of the patients had more than two exacerbations and 13% had at least one severe exacerbation during this first year of follow-up.
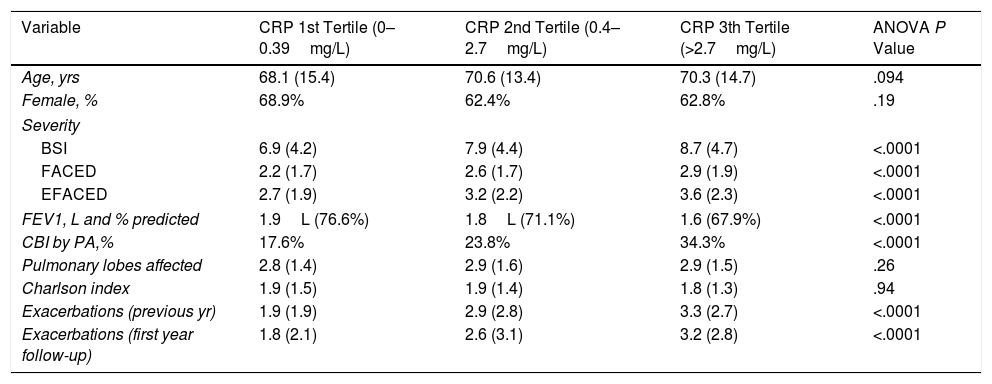
Table 2 shows the comparative characteristics between patients depending on the CRP concentration (in tertiles). Those patients who presented in its third tertile presented more severity, worse lung function, increased percentage of CBI by PA and higher number of exacerbations.
Comparative Characteristic of the Included Patients Depending on the CRP Concentration.
| Variable | CRP 1st Tertile (0–0.39mg/L) | CRP 2nd Tertile (0.4–2.7mg/L) | CRP 3th Tertile (>2.7mg/L) | ANOVA P Value |
|---|---|---|---|---|
| Age, yrs | 68.1 (15.4) | 70.6 (13.4) | 70.3 (14.7) | .094 |
| Female, % | 68.9% | 62.4% | 62.8% | .19 |
| Severity | ||||
| BSI | 6.9 (4.2) | 7.9 (4.4) | 8.7 (4.7) | <.0001 |
| FACED | 2.2 (1.7) | 2.6 (1.7) | 2.9 (1.9) | <.0001 |
| EFACED | 2.7 (1.9) | 3.2 (2.2) | 3.6 (2.3) | <.0001 |
| FEV1, L and % predicted | 1.9L (76.6%) | 1.8L (71.1%) | 1.6 (67.9%) | <.0001 |
| CBI by PA,% | 17.6% | 23.8% | 34.3% | <.0001 |
| Pulmonary lobes affected | 2.8 (1.4) | 2.9 (1.6) | 2.9 (1.5) | .26 |
| Charlson index | 1.9 (1.5) | 1.9 (1.4) | 1.8 (1.3) | .94 |
| Exacerbations (previous yr) | 1.9 (1.9) | 2.9 (2.8) | 3.3 (2.7) | <.0001 |
| Exacerbations (first year follow-up) | 1.8 (2.1) | 2.6 (3.1) | 3.2 (2.8) | <.0001 |
BSI: Bronchiectasis Severity Index; CBI: Chronic Bronchial Infection; PA: Pseudomonas aeruginosa; CRP: C-Reactive Protein.
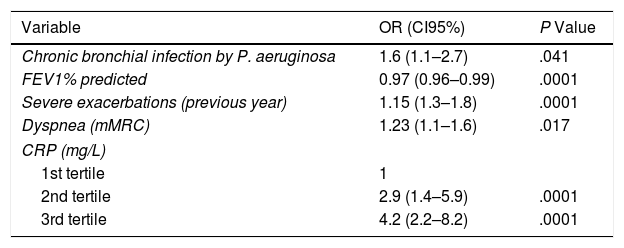
The multivariate logistic regression analysis (Table 3) shows the variables independently associated with an increase in the risk of severe exacerbations in the future. There was a linear relationship between the tertiles of rising values of CRP and a higher risk of severe exacerbations. Thus, the tertile with the greatest concentration presented a 4.2 times greater risk of presenting a severe exacerbation in the first year of the follow-up than the control group (first tertile). No prognostic capacity was observed in gender (OR 0.7 [95%CI: 0.5–1.3]), radiological extension (OR 1.1 [0.88–1.4]), the Charlson Index (OR 1.1 [0.9–1.2]), or any of the treatments analyzed.
Risk Factors for the Presence of at Least One Severe Exacerbation in the First Year of the Follow-up.
| Variable | OR (CI95%) | P Value |
|---|---|---|
| Chronic bronchial infection by P. aeruginosa | 1.6 (1.1–2.7) | .041 |
| FEV1% predicted | 0.97 (0.96–0.99) | .0001 |
| Severe exacerbations (previous year) | 1.15 (1.3–1.8) | .0001 |
| Dyspnea (mMRC) | 1.23 (1.1–1.6) | .017 |
| CRP (mg/L) | ||
| 1st tertile | 1 | |
| 2nd tertile | 2.9 (1.4–5.9) | .0001 |
| 3rd tertile | 4.2 (2.2–8.2) | .0001 |
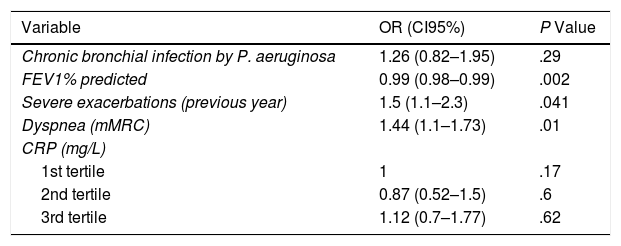
However, the values of the concentration of CRP were not associated with any increase in the future risk of mild-moderate exacerbations, as shown in Table 4.
Risk Factors for the Presence of at Least Two Mild or Moderate Exacerbations in the First Year of the Follow-up.
| Variable | OR (CI95%) | P Value |
|---|---|---|
| Chronic bronchial infection by P. aeruginosa | 1.26 (0.82–1.95) | .29 |
| FEV1% predicted | 0.99 (0.98–0.99) | .002 |
| Severe exacerbations (previous year) | 1.5 (1.1–2.3) | .041 |
| Dyspnea (mMRC) | 1.44 (1.1–1.73) | .01 |
| CRP (mg/L) | ||
| 1st tertile | 1 | .17 |
| 2nd tertile | 0.87 (0.52–1.5) | .6 |
| 3rd tertile | 1.12 (0.7–1.77) | .62 |
Moreover, other variables such as the degree of dyspnea, lung function, and previous severe exacerbations did present a significant prognostic value for future exacerbations, independently of the severity of the exacerbation. However, CBI by PA, like the CRP value, was only associated with severe exacerbations.
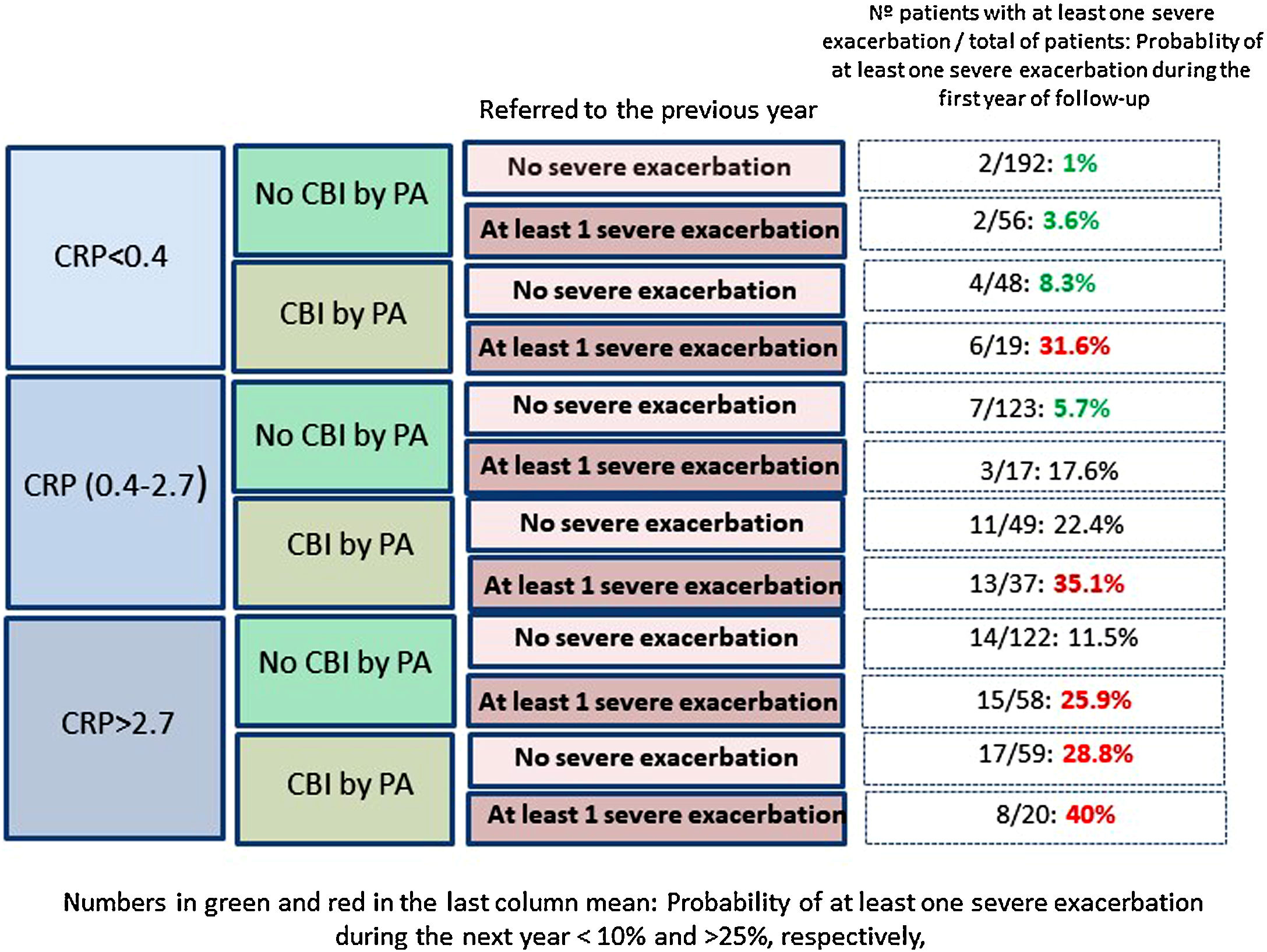
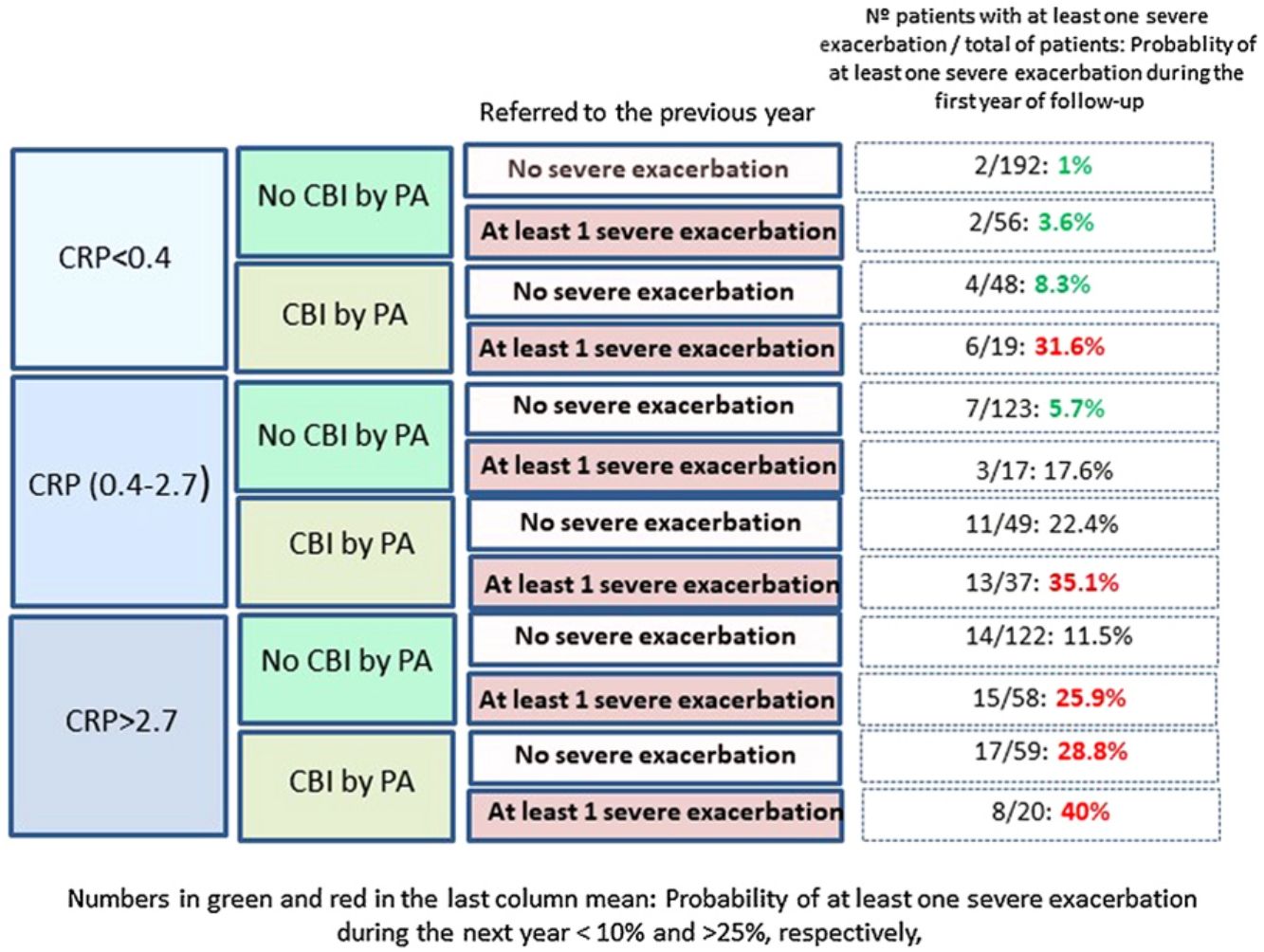
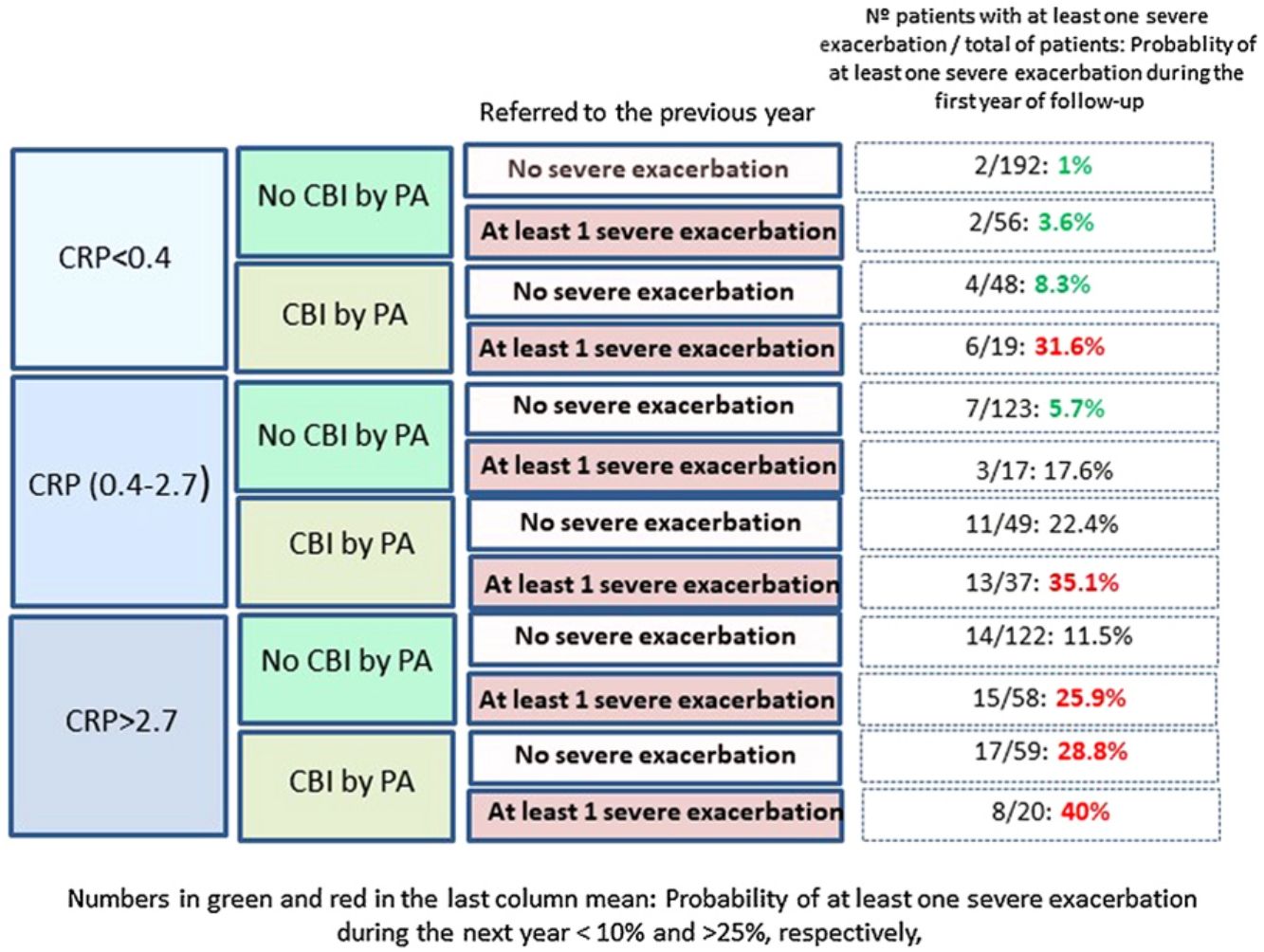
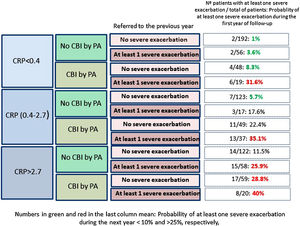
Fig. 2 shows that those patients who presented CPR in its third tertile, CBI by PA and at least one severe exacerbation in the previous year presented a 40% probability of presenting at least one severe exacerbation in the coming year; in contrast, this probability was 1% in patients with none of these three characteristics (OR: 51.5 [CI95%:11–242]).
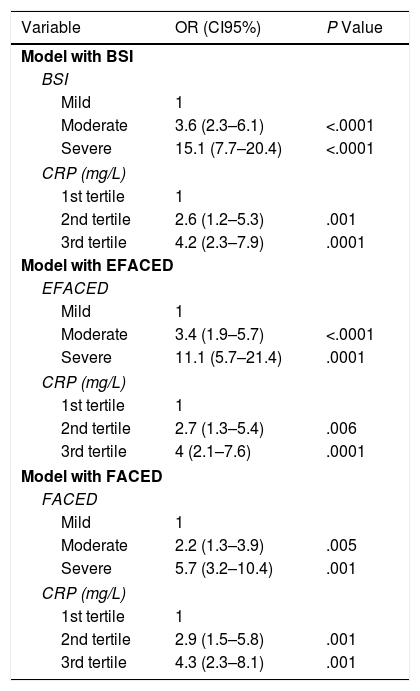
Finally, Table 5 shows how the prognostic capacity for at least one severe exacerbation in the first year of follow-up of the value of the CRP concentration, measured in accordance with the usual multidimensional scales, remained constant regardless of the severity of the BE.
Prognostic Values for at Least One Severe Exacerbation in the First Year of Follow-up of the Levels of CRP, According to the Various Scales of Bronchiectasis Severity.
| Variable | OR (CI95%) | P Value |
|---|---|---|
| Model with BSI | ||
| BSI | ||
| Mild | 1 | |
| Moderate | 3.6 (2.3–6.1) | <.0001 |
| Severe | 15.1 (7.7–20.4) | <.0001 |
| CRP (mg/L) | ||
| 1st tertile | 1 | |
| 2nd tertile | 2.6 (1.2–5.3) | .001 |
| 3rd tertile | 4.2 (2.3–7.9) | .0001 |
| Model with EFACED | ||
| EFACED | ||
| Mild | 1 | |
| Moderate | 3.4 (1.9–5.7) | <.0001 |
| Severe | 11.1 (5.7–21.4) | .0001 |
| CRP (mg/L) | ||
| 1st tertile | 1 | |
| 2nd tertile | 2.7 (1.3–5.4) | .006 |
| 3rd tertile | 4 (2.1–7.6) | .0001 |
| Model with FACED | ||
| FACED | ||
| Mild | 1 | |
| Moderate | 2.2 (1.3–3.9) | .005 |
| Severe | 5.7 (3.2–10.4) | .001 |
| CRP (mg/L) | ||
| 1st tertile | 1 | |
| 2nd tertile | 2.9 (1.5–5.8) | .001 |
| 3rd tertile | 4.3 (2.3–8.1) | .001 |
BSI: Bronchiectasis Severity Index; CRP: C-Reactive Protein.
According to the results of our study, which was carried out in a large, well-characterized cohort of BE patients taken from the RIBRON, peripheral CRP concentration presented a significant independent prognostic value for future severe exacerbations, but not for mild-moderate exacerbations. This risk presented a linear relationship, so the higher the CRP concentration, the greater the risk of a severe exacerbation. We believe that this finding, could have an important application in clinical practice, as it allows a clinician to predict which patients (while free of an exacerbation process) will have the greatest risk of experiencing a severe exacerbation of their disease in the future; this knowledge would therefore make it possible to anticipate any necessary preventive and therapeutic measures.
Exacerbations, particularly severe ones, are a key factor when it comes to evaluating the severity or prognosis of BE. It is hardly surprising, therefore, that they appear, with a relatively substantial weight, in some of the recently published multidimensional scores on BE, such as the Bronchiectasis Severity Index (BSI)29 and the E-FACED,30 and they have been associated with a more marked decline in lung function,9 as occur in other airway diseases.32 This implies that these easily measurable factors that prognosticate which patients are more susceptible to presenting frequent or severe exacerbations could be extremely useful in clinical practice.
It is well-established that a considerable percentage of BE patients present a degree of systemic inflammation, as measured by the peripheral concentration of molecules such as neutrophilic elastase.33 The most widely accepted hypothesis is that these patients present a more severe form of the disease, as the appearance of this systemic inflammation has been associated with a greater degree of local inflammation and with various factors associated with a poor prognosis.18–20 However, although systemic inflammation has been widely studied as a prognostic factor for exacerbations in other airway diseases, such as COPD33,34 and CF,36,37 very little information is available in this respect on BE, particularly as regards CRP. In fact, there has only been one small retrospective study in 69 patients, which found that a CRP concentration >4.26mg/L was significantly associated with a higher percentage of patients with at least two hospitalizations in the previous year. Only idiopathic BE was included, however, and the small number of patients makes it impossible to draw any valid conclusions.28
Our results point in the same direction as studies undertaken on COPD and CF, as a higher peripheral concentration of CRP was associated with a higher risk of future exacerbations, after adjusting the results for variables with a proven predictive value or associated with exacerbations in BE.32–35 In fact, a CRP value between 0.4 and 2.7mg/L, or higher than 2.7mg/L, multiplied by 2.9 and 4.2 times, respectively, the risk of a severe exacerbation in the following year, compared to the control group. If this high risk of severe exacerbations in association with a high CRP value is combined with other clinically significant high-risk variables, such as the presence of a CBI by PA or previous severe exacerbations (both variables with a known prognostic value for exacerbations of BE), the resulting information could be clinically relevant, as shown in Fig. 2. For example, those patients with a CRP >2.7mg/L and a CBI by PA in a clinically stable phase who had had at least one hospitalization in the previous year presented a 51 times greater probability of a future hospitalization than those who did no present any of these three characteristics (40% of possibilities versus 1%).
Furthermore, this prognostic capacity of CRP values with respect to future severe exacerbations was independent of the severity of BE as evaluated by traditional scores, which indicates that this easily obtainable and interpretable biomarker could provide prognostic data that would complement the information already provided by these scores, particularly the BSI and E-FACED.
It should be noted, however, that the CRP concentration did not present any prognostic value for mild-moderate exacerbations. This finding could mean that CRP is indeed exclusively a marker of severe forms of exacerbation, but it could also mean that some mild-moderate exacerbations could have passed unnoticed or unrecorded in the registry, thereby rendering the analysis less trustworthy in this respect.
Two of the strengths of our study are undoubtedly the considerable number of patients included and their good characterization, reflecting their presence on a national registry with strict quality control measures. Another strength is the study's multicenter nature, as it involved 43 different centers from the whole of Spain. The study also presents some limitations, however. On the one hand, only 42.8% of the total number of patients could be analyzed, largely because many did not reach the end of the first year of the follow-up – although the latter's baseline characteristics were analyzed and did not diverge from those of the patients who were finally included. On the other hand, it is well-known that the CRP value is very unspecific since, as an acute-phase reactant, it can produce a high reading in many patients as a result of a number of inflammatory or infectious diseases.20 This is why great caution was taken to extract patients’ blood when they were in a clinically stable state with respect to both BE and any other disease that could alter their levels of CRP, beyond the etiology of BE itself. Finally, since this is data from a non-controlled source, there are bias that cannot be ascertained such as differences in the classification of exacerbations between or even within centers.
In conclusion, CRP concentration, especially above 2.7mg/L, is a good prognostic marker of severe exacerbations in patients with BE, regardless of its severity. As CRP is readily available and easy to measure, its evaluation in conjunction with other clinically relevant variables can enable clinicians to make an early identification of patients with a greater probability of severe exacerbations in the future and implement any necessary preventive and therapeutic measures. Future studies based on other national or international registries are required for the external validation of our results.
Role of SponsorsDevelopment of electronic databases, costs of statistical analysis, and translation of the manuscript. There was no intervention by the sponsor in the design of the study, collection and analysis of data, or writing of the manuscript.
Authors’ Contributions to Study- •
Concept and design: TP and MAMG
- •
Acquisition of data: GO, CV, YD, RG, CO, LM, MGC, OS, RG, JR, EB,
- •
JLR, RM, CP, DR
- •
Drafting of manuscript and critical revision of major intellectual content: All authors.
- •
Final approval of the version to be published: All authors.
RIBRON has a grant from Zambon-Pharma.
Conflict of InterestThe authors declare that there is no conflict of interest regarding the publication of this article.
Guarantor statement: Miguel Angel Martinez-Garcia is the guarantor of the content of the manuscript, including the data, the analysis, and the registry.
Annie Navarro Rolon. Hospital Mutua Terrassa. Barcelona; Patricia Minguez. Hospital Puerta de Hierro. Madrid; Rosanel Amaro. Hospital Clinic. Barcelona; Angela Cervera. Hospital General. Valencia; Marina Blanco. Hospital A Coruña; Ainhoa Gomez. Hospital Cruces. Bilbao; Eleuterio Llorca. Hospital de Elda. Alicante; Alicia Padilla. Hospital de Marbella. Malaga; Edmundo Rosales. Hospital General de Cataluña. Barcelona; Laura Carrasco. Hospital Virgen del Rocio. Sevilla; Marcelo Razquin. Fundació Hospital de Nens. Barcelona.