Congenital tracheal stenosis is a very rare abnormality that accounts for less than 1% of congenital cardiovascular abnormalities and is associated in some cases with other respiratory, esophageal, or skeletal malformations1,2.
Luminal narrowing is caused by the presence of complete or near-complete cartilaginous rings that can occur in 3 patterns: segmental involvement (50% of cases of tracheal stenosis), generalized stenosis (30%), or infundibular stenosis (20%, often related to the anomalous origin of the left pulmonary artery [pulmonary sling], or other vascular abnormalities)3.
Age at onset and clinical severity depend on the degree of stenosis; complete stenosis appears in the neonatal period, and other patterns develop later. Typical clinical manifestations include respiratory distress, cyanosis, dysphagia or difficulty with ingestion, and stridor4. In older patients, it may manifest as repeat pneumonias.
Tracheal narrowing may occasionally be seen on chest X-ray, but the diagnostic method of choice is fiberoptic bronchoscopy, while CT and MRI are useful for defining stenotic extension5,6.
Treatment requires surgical correction7, and the conventional options include laryngotracheal reconstruction, slide tracheoplasty, and partial cricotracheal resection8–11. Less invasive procedures such as balloon dilatation, implantation of endoluminal stents in the area of stenosis, or laser treatment are also available.
Historically, prognosis depended on the extension of the stenosis, but surgical advances have improved morbidity and mortality in patients with severe involvement12.
We report the cases of 2 patients aged 2 and 4 months who were admitted to the Pediatric Intensive Care Unit (PICU) of our hospital with symptoms of respiratory failure in the context of respiratory syncytial virus (RSV) bronchiolitis. Their ventilatory support requirements increased significantly and were difficult to manage appropriately.
The first patient, who had a post-natal diagnosis of trisomy 21, monitored by cardiology for patent ductus arteriosus and by nephrology for left pelvic ectasia, was admitted to the PICO at 2 months of age for respiratory failure due to RSV bronchiolitis. His initial progress on non-invasive ventilation (NIV) with a combination of helium and oxygen was good, but he later required endotracheal intubation and invasive mechanical ventilation (IMV).
The second patient was a late premature twin (35 + 4 weeks gestation). At 4 months of life he presented respiratory failure due to bronchiolitis caused by RSV, which required admission to the PICU. He initially received NIV, with progressive failure and need for IMV.
Both patients were initially connected to bi-level IMV (intermittent mandatory mode) delivered via a face mask (the first initially in continuous pressure with helium-oxygen) but showed progressive clinical and blood gas deterioration despite support optimization, so intubation and connection to IMV were required. Both patients were difficult to intubate, with resistance to correct endotracheal tube (ETT) advancement due to their small endoluminal caliber.
They presented a predominantly obstructive mixed pattern, requiring IMV in volume control mode, with difficult-to-manage ventilatory status despite optimization of support and intensification of bronchodilator treatment (nebulized salbutamol and ipratropium bromide, corticosteroids, intravenous theophylline and magnesium sulphate, sedation and analgesia, and muscle relaxation). They had very high peak inspiratory pressures (PIP) (up to 90 cmH2O), which made it difficult to achieve adequate tidal volumes, despite ventilating without PIP limit, resulting in respiratory acidosis with severe hypercapnia (pCO2 > 150 mmHg), with moments of intermittent improvement.
In light of their slow progress, the original diagnostic work-up was complemented with fiberoptic bronchoscopy and pulmonary CT angiogram. In the second patient, stenosis was not observed in the first fiberoptic bronchoscopy that was performed immediately after intubation, in view of the significant diagnostic suspicion generated by the difficulty of intubation.
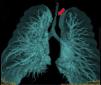
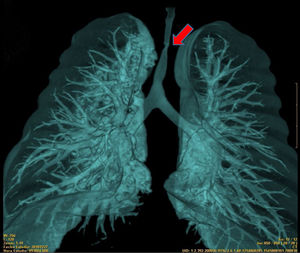
The first patient presented distal tracheal stenosis due to compression of the brachiocephalic artery, with a critical diameter of 1 mm (Fig. 1). The second patient had distal infundibular tracheal stenosis of 90% of the tracheal lumen, 5 cm in length with a critical diameter of 1 mm in the supracarinal region and 4−5 mm in the cervical trachea.
Both patients underwent slide tracheoplasty at the reference hospital for airway diseases. The first patient made good post-surgical progress. The second patient initially underwent slide tracheoplasty of the entire length of the trachea (except the first and second rings), with reimplantation of the left pulmonary branch. He subsequently required several reinterventions (balloon dilations and placement of 2 tracheal endoluminal prostheses). Finally, tracheostomy was performed with subsequent partial tracheal resection due to recurrent peristomal granulomas.
A critical situation in patients with tracheal stenosis is usually triggered by an inflammatory component associated or not with mucous plugs that worsen existing narrowing, as in our patients, due to bronchiolitis caused by RSV. It is often possible to intubate these patients, but difficulty can be encountered when advancing the tube distally to the glottis. Technological developments that offer 3-dimensional airway reconstructions have facilitated the development of “virtual tracheobronchoscopy”, a significant advance in the diagnosis of tracheobronchial anomalies13,14. The underlying diagnostic suspicion arises at the time of intubation and usually persists in the presence of a severe obstructive pattern that hinders ventilatory support. However, this severity may be intermittent depending on the depth of ETT fixation relative to the extent of the lesion, as observed in both patients.
Affected patients, when clinically destabilized, usually require IMV for a median duration of 59 days15. Some studies have analyzed various parameters to evaluate predicted survival, and conclude that neither the duration of ventilatory support nor the extent of stenosis measured by dynamic contrast bronchoscopy are good predictive tools15.
In PICU patients with slow-progressing obstructive respiratory disease and difficult ventilatory management, fiberoptic bronchoscopy may be useful to rule out hitherto asymptomatic congenital airway malformations and possible complications of intensive and prolonged respiratory support (granulomas, stenosis, vocal cord paralysis).
As congenital tracheal stenosis is rare, a high degree of suspicion is needed to complete and establish the diagnosis. Management is complex and requires an individualized approach by expert multidisciplinary teams working in reference units. The treatment is surgical, and outcomes can vary depending on the techniques used and the series described.
Ventilatory support of these patients in the PICU is challenging. When the extent of the lesion exceeds the length of the TEE, it causes a severe obstructive pattern, and ventilatory support can be very complicated and intensive.
FundingNone.
Conflict of interestsNone.
Please cite this article as: Baquedano Lobera I, Gil Hernández I, Madurga Revilla P. «Bronquiolitis inventilables» como manifestación de estenosis traqueales congénitas. Arch Bronconeumol. 2021;57:660–661.