Tuberculous pleural effusion (TBPE) is the most common form of extrapulmonary tuberculosis (TB) in Spain, and is one of the most frequent causes of pleural effusion. Although the incidence has steadily declined (4.8 cases/100000 population in 2009), the percentage of TBPE remains steady with respect to the total number of TB cases (14.3%–19.3%). Almost two thirds are men, more than 60% are aged between 15 and 44years, and it is more common in patients with human immunodeficiency virus. The pathogenesis is usually a delayed hypersensitivity reaction. Symptoms vary depending on the population (more acute in young people and more prolonged in the elderly). The effusion is almost invariably a unilateral exudate (according to Light's criteria), more often on the right side, and the tuberculin test is negative in one third of cases.
There are limitations in making a definitive diagnosis, so various pleural fluid biomarkers have been used for this. The combination of adenosine deaminase and lymphocyte percentage may be useful in this respect. Treatment is the same as for any TB. The addition of corticosteroids is not advisable, and chest drainage could help to improve symptoms more rapidly in large effusions.
El derrame pleural tuberculoso (DPTB) es la causa más frecuente de tuberculosis (TB) extrapulmonar en nuestro país y uno de los motivos más habituales de derrame pleural. Si bien la incidencia disminuye progresivamente (4,8 casos/100.000 habitantes en el año 2009), el porcentaje de DPTB se mantiene estable con respecto al número de casos totales de TB (14,3-19,3%). Casi las dos terceras partes son hombres, más del 60% tienen edades entre los 15-44años y es más frecuente en los pacientes infectados por el virus de la inmunodeficiencia humana. La patogenia suele ser una reacción de hipersensibilidad retardada. La clínica varía dependiendo de la población (más aguda en los jóvenes y más prolongada en los ancianos). El derrame es casi invariablemente un exudado unilateral (según los criterios de Light), más frecuentemente del lado derecho, y la prueba de la tuberculina es negativa en la tercera parte de los casos.
Los diagnósticos de certeza tienen limitaciones, por lo que para ello se han utilizado diversos biomarcadores en el líquido pleural. La asociación de la adenosina desaminasa y del porcentaje de linfocitos puede ser útil para el diagnóstico. El tratamiento es el de cualquier TB. No parece recomendable añadir corticoides y el drenaje torácico podría contribuir, en los grandes derrames, a una mejoría más rápida de los síntomas.
Tuberculosis (TB) is one the greatest causes of morbidity and mortality worldwide due to infection, and public health concerns are very real. The World Health Organization estimated that there were 8.7 million new cases of TB in 2011, equivalent to a global incidence of 125/100000individuals/year, while 1.4 million sufferers died.1 TB and human immunodeficiency virus (HIV) co-infection together with immigration have led to a resurgence of TB in developed countries2 and add to the disease burden in developing countries.1 Tuberculous pleural effusion (TBPE) is widespread in many countries,3 and in clinical practice, it is most often found in the context of HIV infection.4
EpidemiologyIn Galicia, where the authors practice, 690 new cases of TB were registered in 2011 (incidence, 24.6/100000inhabitants/year),5 while in the whole of Spain 6746 cases (incidence, 14.6/100000inhabitants/year)6 were diagnosed. In Galicia, TBPE is the most common cause of extrapulmonary TB.7 In 2011, 109 new cases were diagnosed, comprising 15.8% of the total cases of TB registered.5 From an epidemiological point of view, TBPE is declining significantly: between 2000 and 2009, both the number of cases and the incidence in our community fell by half (262–133 and 9.6%–4.8%, respectively).7 However, the percentage of TBPE patients out of the total number of TB cases has not changed significantly (between 14.3% and 19.3%),8 far greater than in the United States (3.6%),9 but lower than in some African countries, where rates of over 20% have been reported.10,11 These differences can be attributed to varying prevalence of TB among the general population,12 or, as pleural fluid (PF) culture is generally negative, the incidence of TBPE is perhaps underestimated.13 TBPE is more common in men (63.5%),8 in patients aged 15–44 years (61.2%)7, and in HIV-positive patients.8,10,11 Although a higher incidence might be expected in immunocompetent patients than in those with cell-mediated immunity disorders, this is generally not the case.10,11,14,15
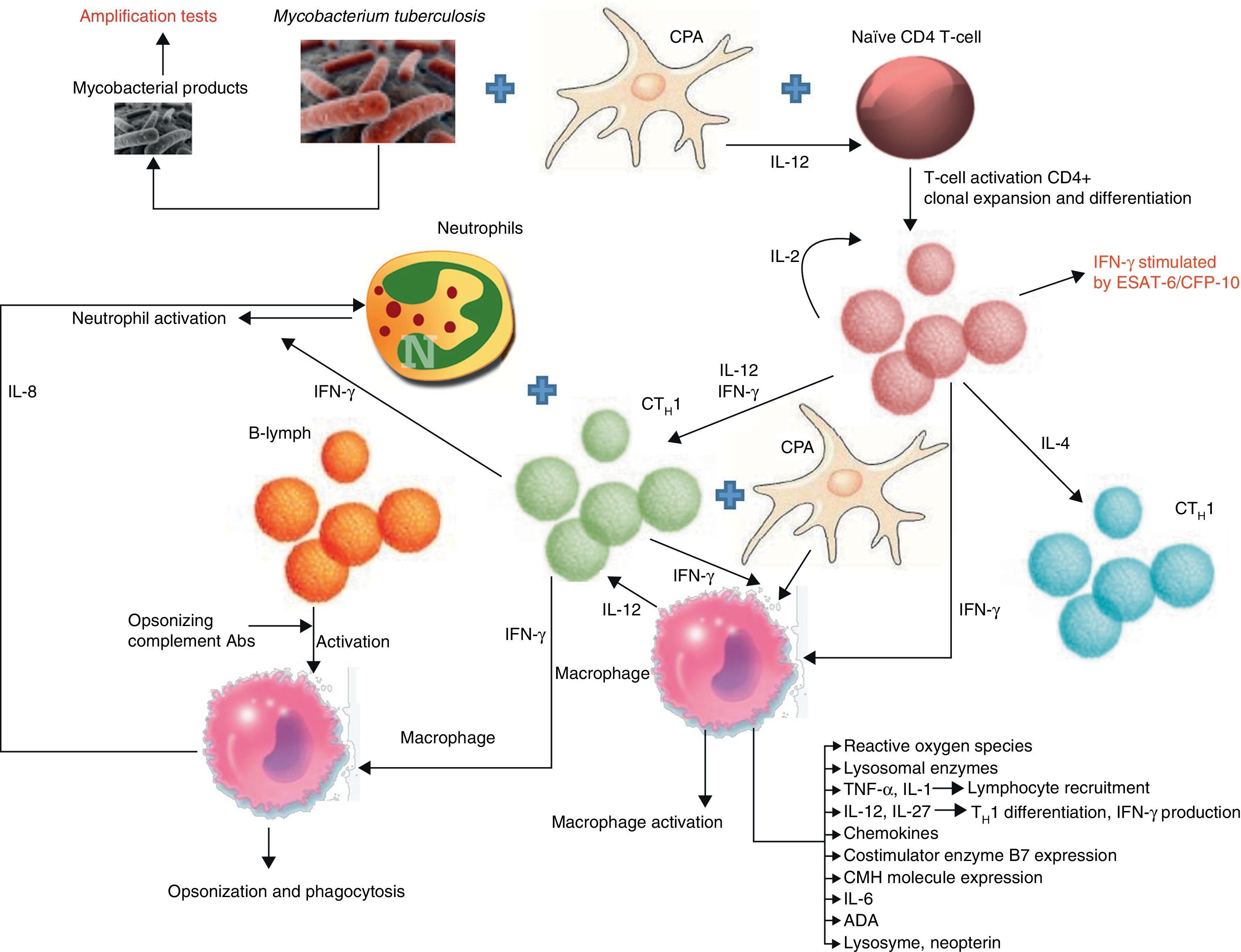
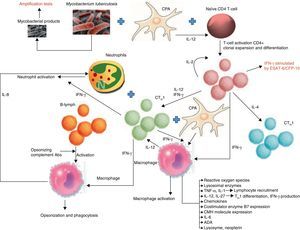
PathogenesisTBPE can be a manifestation of both primary infection and disease reactivation. The latter is the most common in developed countries.16 TBPE is thought to occur when a subpleural caseous focus ruptures releasing its contents into the pleural space.17 Mycobacterial antigens interact with CD4+ T-lymphocytes, leading to a delayed hypersensitivity reaction in which different cytokines stimulate macrophage antimycobacterial activity (Fig. 1). The resulting increased capillary permeability and diminished lymphatic drainage causes pleural effusion (PE). This theory is based on the fact that PF culture in these patients18 is generally negative, yet the injection of tuberculous protein into the pleural space of guinea pigs previously sensitized with purified protein derivatives produces exudative PE.19 When the animals are treated with antilymphocyte serum, the exudative PE resolves.20
Biomarkers and pathways involved in the immunological response of tuberculous pleural effusions. B7: proteins expressed by antigen-presenting cells; CFP: culture filtrate protein; MHC: major histocompatibility complex; APC: antigen-presenting cell; CTH1: T-lymphocyte responsible for cell-mediated or delayed immunity; CTH2: T-lymphocyte responsible for humoral immunity; ESAT: early secreted antigenic target; IFN-γ: interferon gamma; IL: interleukin; B-lym: B-lymphocyte.
Tuberculous empyema is a chronic active infection of the pleural space. It may be a consequence of infection extending from other sites after pneumonectomy, or it can occur when the contents of a bronchopleural fistula cavity are released into the pleural space.21
TBPE can also cause fibrous thickening of the visceral pleura, preventing lung expansion (trapped lung). Negative pressure is created in the pleural space, and chronic PE, with all its typical features, can occur even if the TB disease is inactive.22
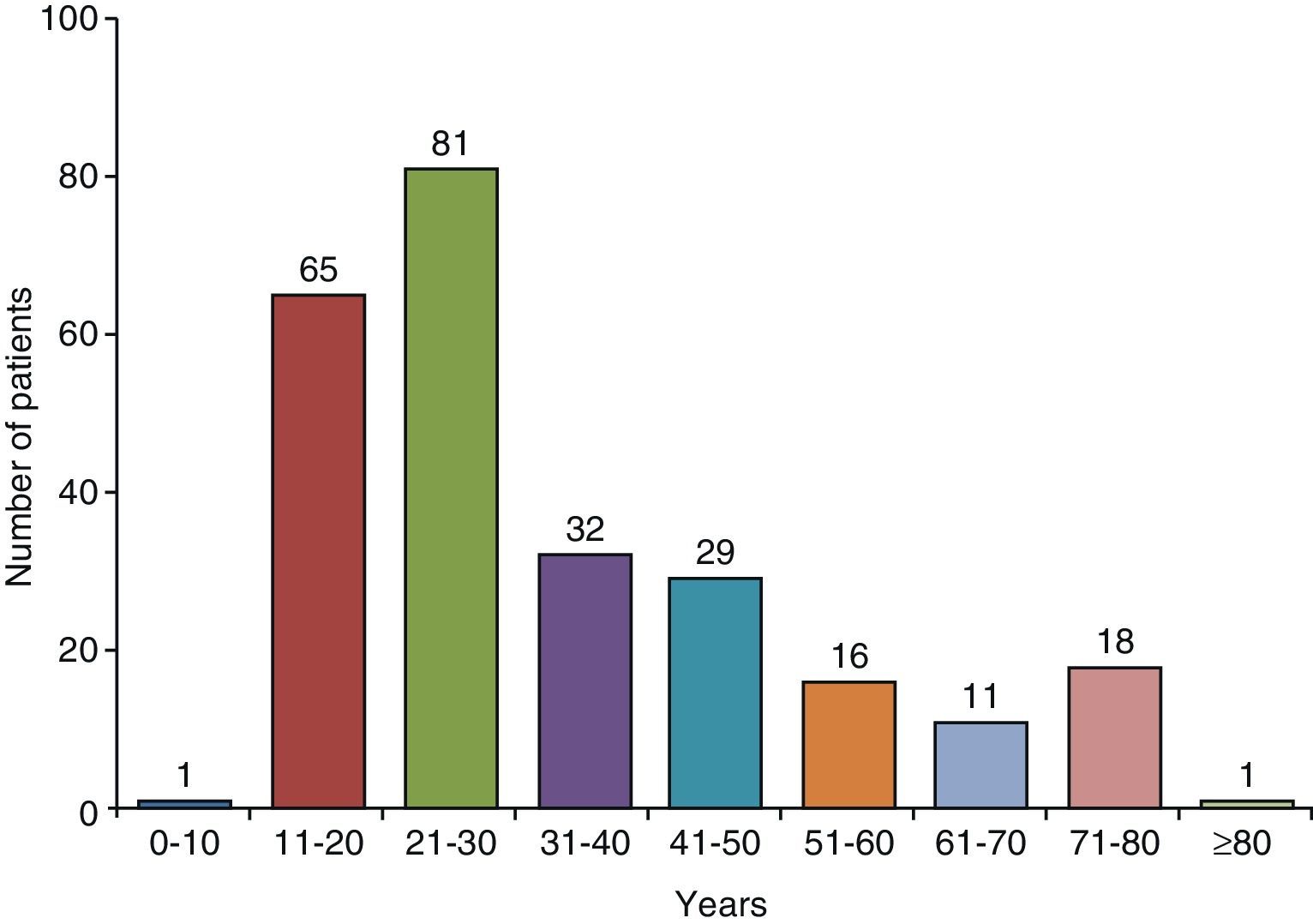
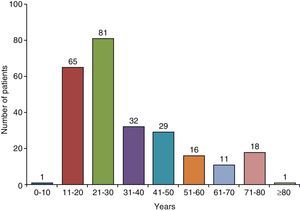
Clinical CharacteristicsIn countries with high TB incidence, the mean age of TBPE patients ranges from 32 to 34 years23–27 and 70% are under the age of 408,24 (Fig. 2). In the United States, mean age at presentation is 49; only 50% of patients are under 45, and 30% are over 65 years of age.9 To explain these differences, it has been suggested that in developed countries, TBPE is the result of reactivation, while in countries where it appears in a younger population, it is a primary form of the disease.16
Age distribution of patients with tuberculous pleural effusion in Galicia (Spain).
TBPE symptoms are variable. Fever (86% of cases), pleuritic chest pain (75%), and cough (70%) may be accompanied by other systemic symptoms.28,29 The clinical picture varies depending on the population. The elderly tend to be symptomatic over a prolonged period, while HIV-positive patients generally present fewer symptoms. In contrast, younger patients have more acute clinical symptoms and high fever.30
TBPE is usually unilateral and can be of any size. In our series of 254 cases, 98.5% were unilateral (56% on the right side). Massive TBPEs are unusual but, even so, comprise the third most common presentation.31 Lung involvement is seen on chest X-ray in 25% of cases,8 but this rate may be higher than 50% if chest computed tomography (CT) is used.32 The percentage of lung involvement depends on whether TBPE is a consequence of primary infection or disease reactivation (in which case it will presumably be higher). A third of TBPEs will be Mantoux-negative. In these cases, the test should be repeated after 2–6 weeks since it may become positive later17 and could provide further confirmation of the diagnosis. The HIV-positive patient group, particularly those with a CD4+ count <200cells/mm3, will yield more Mantoux-negative cases.33
DiagnosisThe definitive diagnosis of TBPE depends on the demonstration of tuberculous bacilli in sputum, PF or pleural biopsy (PB) specimens, or the observation of granulomas in the latter.34
MicrobiologyA frequently overlooked test in the diagnosis of PE is a sputum culture for mycobacteria. This is positive in between 41.7% and 52% of cases.8,35,36
PF staining for TBPE diagnosis in an immunocompetent patient does not appear to be useful (5% yield).24 However, staining can be positive in 20%37 of HIV-positive patients, and is justified in this population. PF culture yield is higher, depending on the culture medium used. The bacillus is identified in 12%–36.6% of cases24,38 when solid media (Löwenstein–Jensen) is used. Liquid media are more sensitive. Culture yield with the BACTEC system increases from 12% to 24%,38 while Ruan et al.34 achieved 63% with a significantly shorter waiting period.38,39 Two factors may play a part in the result: HIV infection and the predominant cell population in PF. Both PF staining and culture are more often positive in HIV-positive patients than in HIV-negative subjects (37% vs 0% for staining; 43% vs 12% for solid medium culture; 75% vs 24% for liquid medium culture).37,38 In these cases, TBPE may be less due to a reaction to tuberculin proteins and more to reduced mycobactericidal macrophage activity in a weakened immune system, permitting mycobacteria to reside longer in the pleural space. The second factor that could affect culture yield is the predominant cell population. The higher the proportion of neutrophils in PF, the greater the chance that the culture will be positive.35,40 This is probably because in the early stages of the disease, when neutrophils are predominant, the immune system is not yet effective against bacilli. In this situation, the mycobacterial load in PF is greater. When the activated macrophages phagocytize Mycobacterium tuberculosis (MT), the intrapleural mycobacterial load, and therefore the chance that the culture will be positive, is reduced.41
MODS (microscopic-observation drug-susceptibility) culture is a cheap, highly sensitive and relatively rapid liquid medium from which drug susceptibility data can be obtained simultaneously. The yield from this culture in both PF and PB is greater than with Löwenstein–Jensen (20% vs 7% and 81% vs 51%, respectively) and waiting time is shorter.42
As there are few bacilli in TBPE, microbiological methods lack sensitivity. Specific TB nucleic acid sequence amplification techniques are a fast way of detecting MT in PF, even if there are few bacilli.43 In a meta-analysis of 38 articles, sensitivity was 62% and specificity was 98%,44 suggesting that this method is useful for confirming diagnosis but not for ruling it out. Low sensitivity can be attributed to the low bacillus load of the samples, the probable presence of amplification-inhibiting substances in the PF, the amplified genomic sequence, and possible intracellular mycobacterial sequestration. This test is not routinely performed in the determination of PE, due to its poor sensitivity and high cost.
Xpert is an automatic nucleic acid amplification method that simultaneously detects MT and rifampicin resistance in less than 2h.45 However, its TBPE diagnostic yield is low: specificity is 100% but sensitivity ranges between 15% and 27%.46–48
Pleural BiopsyClosed PB is the most sensitive method for diagnosing TBPE. Granuloma are seen in 50%–97% of cases.17,18,24,49–51 Although granulomatous pleuritis occurs with other diseases, over 95% of cases are caused by TB. TBPE diagnosis can be increased from 79.8% without systematic PB culture to 91.5% when this technique is performed.24 Thoracoscopy can also be useful, although it is usually not necessary to go to these lengths. However, the sensitivity of this technique in TB patients can be as high as 100%.52
There are therefore numerous difficulties involved in reaching a definitive diagnoses: low test yields, long waiting time for culture results, invasive techniques such as BP. The value of determining TB immunological response biomarkers in PF for improving diagnostic yield has been evaluated (Fig. 1).
Adenosine DeaminaseAdenosine deaminase (ADA) is a widely distributed enzyme that plays a significant role in T-lymphocyte proliferation and differentiation. A meta-analysis of 63 studies of over 8000 PEs, 2796 of which were TBPE, shows that the sensitivity, specificity, positive and negative probability ratios (PPR and NPR, respectively), and the odds ratio were 92%, 90%, 9, 0.10, and 110, respectively.53 ADA has two isoenzymes: ADA1 and ADA2. The first is found in all cells, while the other is only found in monocytes/macrophages and levels increase when these cells are stimulated by live microorganisms.54 ADA2 is more common in TBPE. There are 3 methods for determining ADA isoenzymes: (a) fraction separation by electrophoresis55,56; (b) erythro-9(2-hydroxy-3-nonyl)adenine (EHNA) ADA1 inhibition,55,57–59 and (c) ADA1 and ADA2 calculation using their different affinity ratios for adenosine and 2′-deoxyadenosine substrates.60–62 The latter yields 2′-deoxyadenosine/adenosine or ADA1/ADA63 ratios, although the first method generates fewer errors, since the data are obtained from enzyme activity and not from theoretical calculations.
Although ADA2 yield is slightly higher (ADA vs ADA2: sensitivity 100% for both, specificity 91% vs 96%60; sensitivity 93.7% vs 97%, specificity 89% vs 94%64), its use in clinical practice does not seem to be justified.
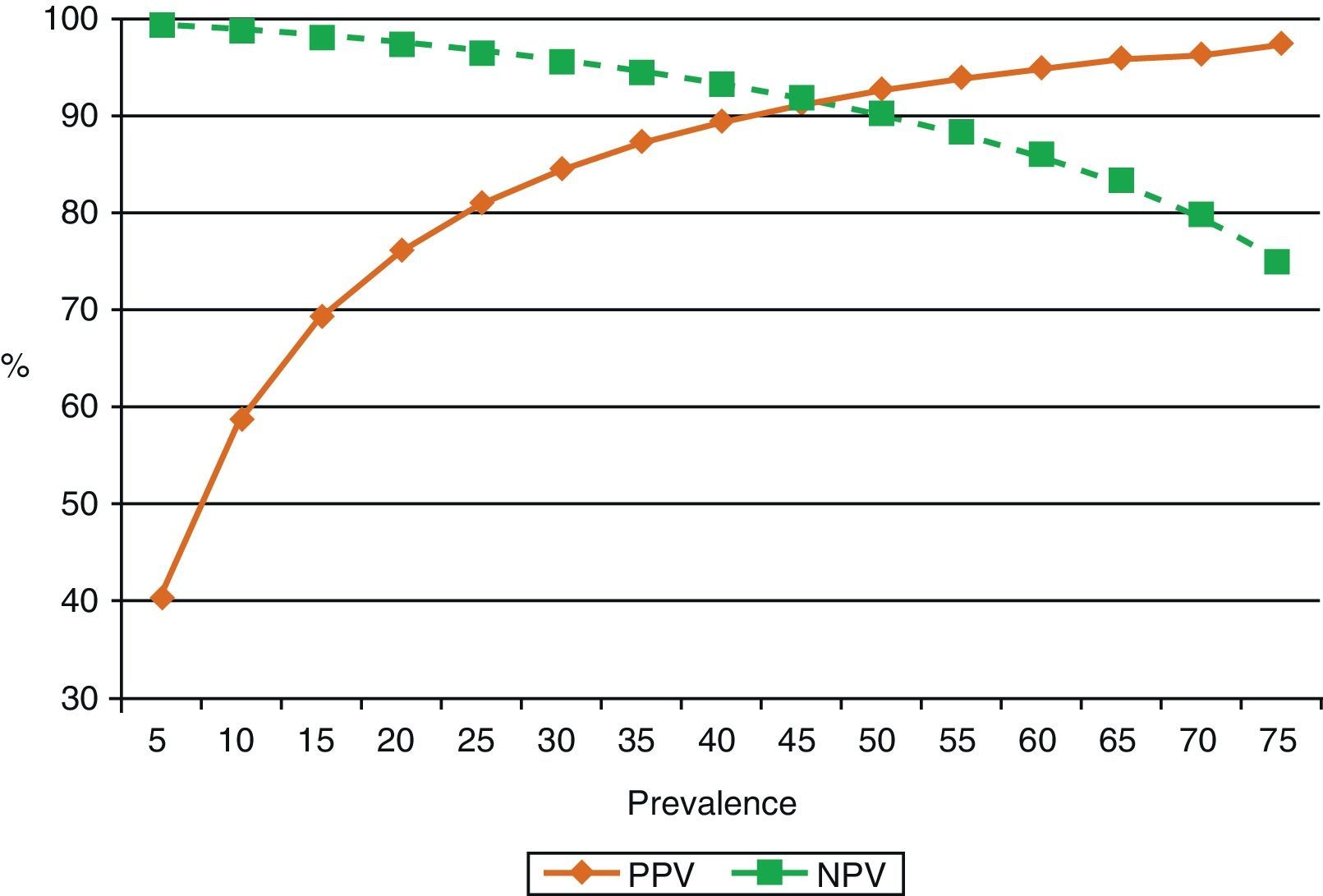
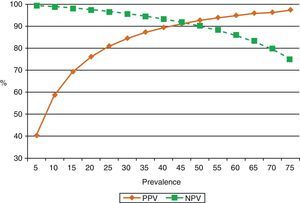
ADA levels rise in TBPE with low CD4+ cell counts65 (sensitivity 94%, specificity 95%). This is probably because monocytes are not affected by retroviral infection, unlike cell populations in which neutrophils predominate.40,66 One reason for this may be that neutrophils release cytokines that attract ADA-2-producing monocytes and macrophages into the pleural space, thus contributing to enhanced ADA activity in these effusions.67 ADA in PF is routinely determined in countries in which the prevalence of TBPE is moderate to high. In these countries, ADA yield in subjects under the age of 35 years is so high that PB may not even be required for diagnosis.68 However, in countries in which prevalence is low, the positive predictive value of this test will be equally low and will only be useful for ruling out the disease (high negative predictive value)69 (Fig. 3).
Effect of prevalence of tuberculous pleural effusion on positive and negative predictive values of diagnostic adenosine deaminase (sensitivity 90%, specificity 93%). When disease prevalence is low, the positive predictive value is also low, so this parameter is useful for ruling out the disease (high negative value). If prevalence is high, the positive predictive value is also high, so it may be useful for confirming the disease. NPV: negative predictive value; PPV: positive predictive value.
ADA determination has several limitations. As various methods are available, results may not be comparable and cutoff points can vary, while other factors, such as ethnicity, may also play a part. The cutoff point, therefore, should be established according to the results of each site, or at least, on the basis of similar population studies using the same methodology.70 In 3 studies of 630 patients with non-TB lymphocytic PE, 2.8% of the cases (18 patients) had high ADA levels.71–73 Raised ADA levels may be found in one third of parapneumonic effusions and in two thirds of empyemas,74 as well as in other types of effusion.23,24 ADA also produces false negatives, but repeatedly low levels seem to rule out the disease.75 ADA remains an inflammatory biomarker and thus cannot be viewed as a substitute for culture nor does it provide information on anti-TB drug sensitivities. In regions with high mycobacterial resistance, therefore, a lymphocytic exudate and a high ADA result would suggest the need for PB, culture, and drug sensitivity testing.76
Interferon GammaInterferon gamma (IFN-γ) is a cytokine released by CD4+ T-lymphocytes that increases macrophage mycobacterial activity. IFN-γ in PF or released by mononuclear cells in PF after stimulation from MT-specific antigens can be determined. In a meta-analysis of 22 articles and 2101 patients (782 with TBPE), sensitivity, specificity, PPR, NPR, and odds ratio were 89%, 97%, 23.45, 0.11, and 272.7, respectively.77 As with ADA, there is no universal cutoff point, because laboratory methods are not always the same. Malignant effusions, particularly if they are hematological, and empyemas may also present high IFN-γ levels.78 IFN-γ yield in TBPE is slightly higher than that of ADA, but the difference is not significant.79
Determination of IFN-γ stimulated with MT-specific antigens (ESAT-6 and CFP-10) may contribute to TBPE diagnosis.80 In a meta-analysis of 366 patients (213 with TBPE) from 7 studies, sensitivity, specificity, PPR, NPR, and odds ratio in PF were 75%, 82%, 3.5, 0.24, and 19, respectively. Levels in blood were slightly lower.81 The quality and cost of these results do not justify routine determination in daily clinical practice.82
Other BiomarkersDetermination of other biomarkers in PF for the diagnosis of TBPE had a lower yield less than that of ADA and IFN-γ (see summary in Table 1).83–94
Other Biomarkers Used in the Diagnosis of Tuberculous Pleural Effusions.
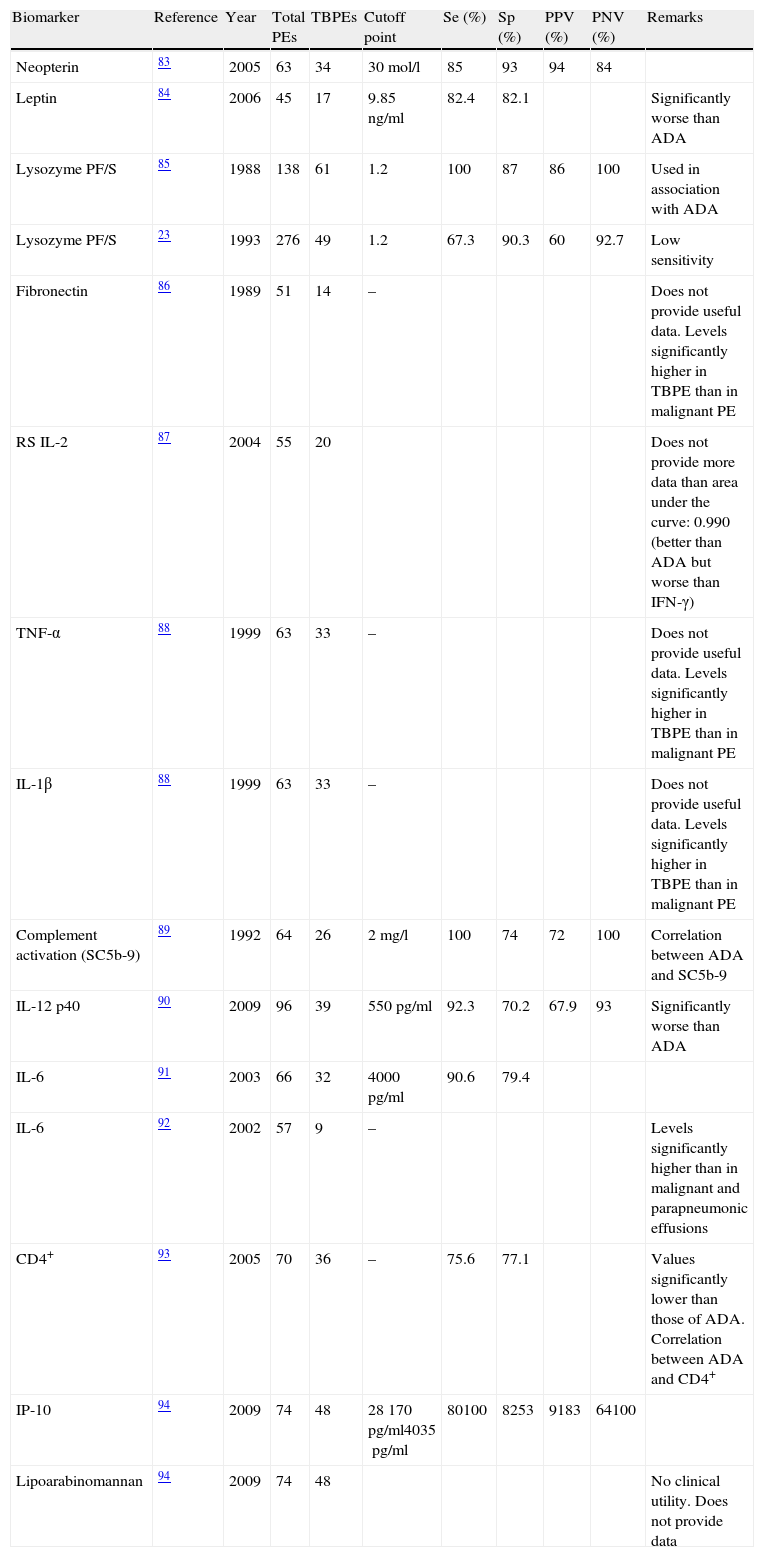
| Biomarker | Reference | Year | Total PEs | TBPEs | Cutoff point | Se (%) | Sp (%) | PPV (%) | PNV (%) | Remarks |
| Neopterin | 83 | 2005 | 63 | 34 | 30mol/l | 85 | 93 | 94 | 84 | |
| Leptin | 84 | 2006 | 45 | 17 | 9.85ng/ml | 82.4 | 82.1 | Significantly worse than ADA | ||
| Lysozyme PF/S | 85 | 1988 | 138 | 61 | 1.2 | 100 | 87 | 86 | 100 | Used in association with ADA |
| Lysozyme PF/S | 23 | 1993 | 276 | 49 | 1.2 | 67.3 | 90.3 | 60 | 92.7 | Low sensitivity |
| Fibronectin | 86 | 1989 | 51 | 14 | – | Does not provide useful data. Levels significantly higher in TBPE than in malignant PE | ||||
| RS IL-2 | 87 | 2004 | 55 | 20 | Does not provide more data than area under the curve: 0.990 (better than ADA but worse than IFN-γ) | |||||
| TNF-α | 88 | 1999 | 63 | 33 | – | Does not provide useful data. Levels significantly higher in TBPE than in malignant PE | ||||
| IL-1β | 88 | 1999 | 63 | 33 | – | Does not provide useful data. Levels significantly higher in TBPE than in malignant PE | ||||
| Complement activation (SC5b-9) | 89 | 1992 | 64 | 26 | 2mg/l | 100 | 74 | 72 | 100 | Correlation between ADA and SC5b-9 |
| IL-12 p40 | 90 | 2009 | 96 | 39 | 550pg/ml | 92.3 | 70.2 | 67.9 | 93 | Significantly worse than ADA |
| IL-6 | 91 | 2003 | 66 | 32 | 4000pg/ml | 90.6 | 79.4 | |||
| IL-6 | 92 | 2002 | 57 | 9 | – | Levels significantly higher than in malignant and parapneumonic effusions | ||||
| CD4+ | 93 | 2005 | 70 | 36 | – | 75.6 | 77.1 | Values significantly lower than those of ADA. Correlation between ADA and CD4+ | ||
| IP-10 | 94 | 2009 | 74 | 48 | 28170pg/ml4035pg/ml | 80100 | 8253 | 9183 | 64100 | |
| Lipoarabinomannan | 94 | 2009 | 74 | 48 | No clinical utility. Does not provide data |
ADA: adenosine deaminase; PE: pleural effusion; TBPE: tuberculous pleural effusion; Sp: specificity; IFN-γ: interferon gamma; IP-10: inducible protein 10; PF/S: pleural fluid/serum; S IL-2R: soluble interleukin-2 receptors; S: sensitivity; TNF-α: tumor necrosis factor-α; NPV: negative predictive value; PPV: positive predictive value.
Although differential cell count in the first 2 weeks can reveal a predominantly polymorphonuclear cell population,95 the predominant population is normally leukocytes (>50%).34 If the leukocyte count is higher than 80%, the differential diagnosis would include TB, lymphoma, and some rare cases of metastatic PE.96 Other possibilities can be ruled out due to low prevalence (for example, rheumatoid pleuritis, and sarcoidosis),97 clinical history (aorto-coronary bypass surgery,98 acute lung rejection, and yellow nail syndrome), or the appearance of the fluid (chylothorax). Mesothelial cell count is rarely >5%,99 although a higher figure would not rule out TB.100 The suggestion that a PF eosinophil count >10% all but rules out TB still more controversial.101,102
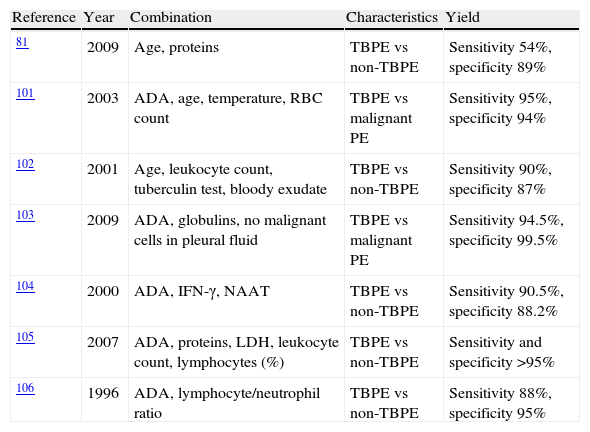
Combined ParametersSeveral studies have developed diagnostic tests based on a combination of variables (Table 2).82,103–108 In general, all manage to improve the yield of individual parameters, but since they rely on complex, often arbitrary, statistical calculations, they are of little use in daily clinical practice. In contrast, Burgess et al.108 obtained a high diagnostic yield with the combination of ADA >50U/l and a lymphocyte/neutrophil ratio of >0.75.
Combination of Variables for the Diagnosis of Tuberculous Pleural Effusions.
| Reference | Year | Combination | Characteristics | Yield |
| 81 | 2009 | Age, proteins | TBPE vs non-TBPE | Sensitivity 54%, specificity 89% |
| 101 | 2003 | ADA, age, temperature, RBC count | TBPE vs malignant PE | Sensitivity 95%, specificity 94% |
| 102 | 2001 | Age, leukocyte count, tuberculin test, bloody exudate | TBPE vs non-TBPE | Sensitivity 90%, specificity 87% |
| 103 | 2009 | ADA, globulins, no malignant cells in pleural fluid | TBPE vs malignant PE | Sensitivity 94.5%, specificity 99.5% |
| 104 | 2000 | ADA, IFN-γ, NAAT | TBPE vs non-TBPE | Sensitivity 90.5%, specificity 88.2% |
| 105 | 2007 | ADA, proteins, LDH, leukocyte count, lymphocytes (%) | TBPE vs non-TBPE | Sensitivity and specificity >95% |
| 106 | 1996 | ADA, lymphocyte/neutrophil ratio | TBPE vs non-TBPE | Sensitivity 88%, specificity 95% |
ADA: adenosine deaminase; TBPE: tuberculous pleural effusion; IFN-γ: interferon gamma; LDH: lactate dehydrogenase; NAAT: nucleic acid amplification test; RBC: red blood cell.
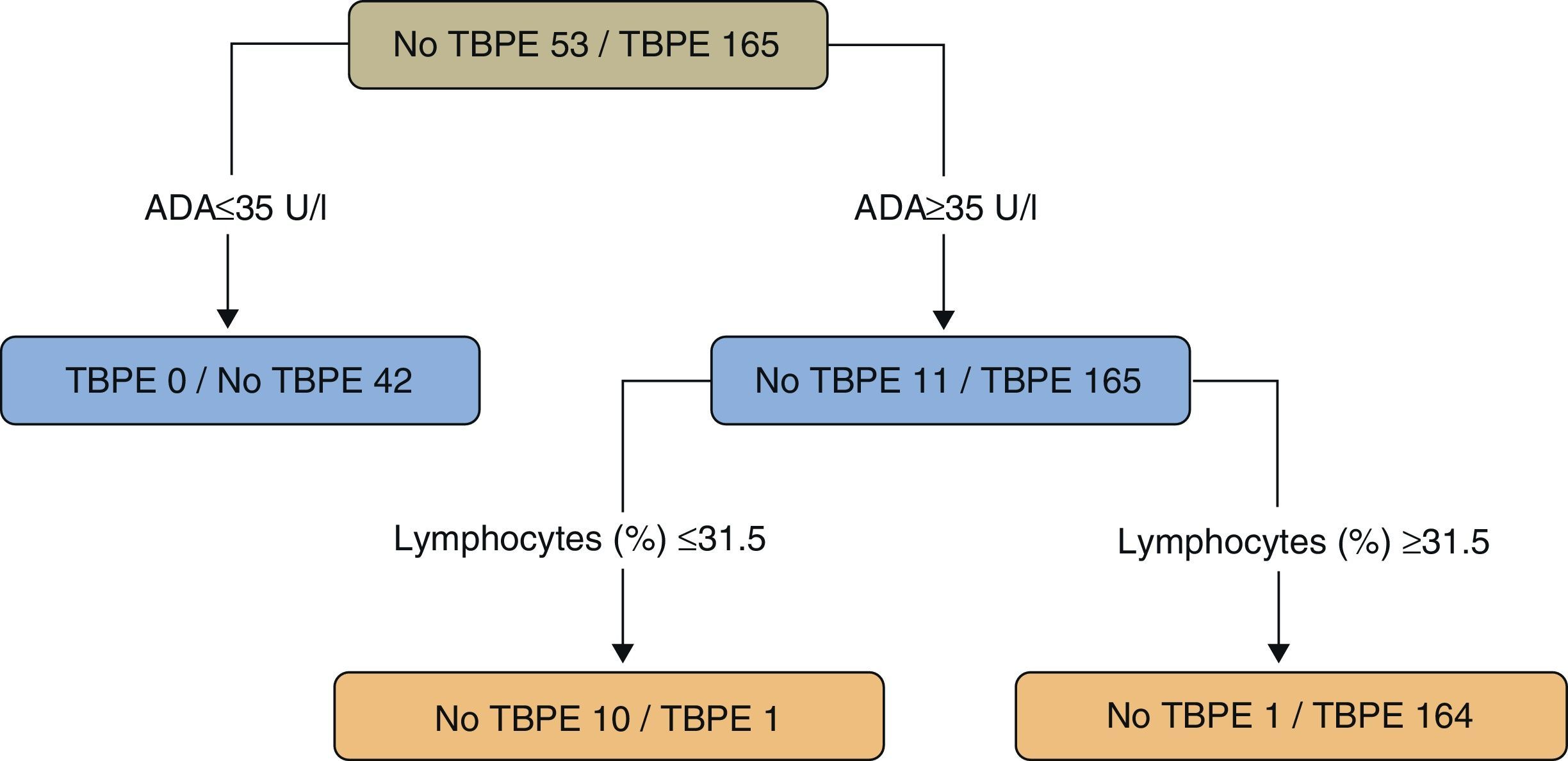
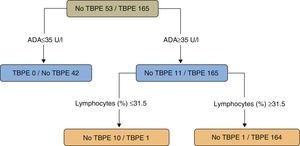
In a study of patients under the age of 40 (218 cases, 165 with TBPE), a regression tree was used to classify PE as TB or non-TB. Twenty variables (clinical, radiological, laboratory tests, and tuberculin results) were taken into consideration, while the statistical program only selected ADA values (as the principal variable) (>35U/l) and the lymphocyte percentage (>31.5%) (sensitivity 99.4%, specificity 98.1%) (Fig. 4).109 The results of a second model, without ADA, selecting (in this order) lymphocyte percentage, fever and cough were significantly worse than the ADA model (sensitivity 95.2%, specificity 94.3%) (P<.001).
Regression tree for predicting tuberculous pleural effusion. Adenosine deaminase (U/l) and lymphocytes (%) were the variables selected for the final regression tree. ADA: adenosine deaminase; TBPE: tuberculous pleural effusion.
In a study designed to determine whether TBPE can be diagnosed solely by PF analyses, ADA >45U/l and lymphocytes >80% had a sensitivity of 58.4% and specificity of 99.5%. Specificity of 100% was achieved if proteins >5g/dl were added to these criteria (sensitivity 34.9%).110
TreatmentUntreated TBPE can resolve spontaneously within 1–4 months, although pulmonary TB may subsequently develop in 65% of cases.111 In accordance with current guidelines, TBPE, like pulmonary TB, should be treated with 4 drugs (isoniazid, rifampicin, pyrazinamide, and ethambutol) for 2 months, and isoniazid and rifampicin for a further 4 months (2HRZE/4HR).112,113 Ethambutol is included if primary resistance to isoniazid is high (>4%).113 If this is not the case and lung involvement is ruled out, an ethambutol-free regimen may be equally useful as PF is paucibacillary.113 Cañete et al.114 reported no treatment failures with 6 months of rifampicin and isoniazid in a series of 130 patients with no lung involvement. These results, however, have not been confirmed in the literature, and the treatment schedule is not accepted in prevailing guidelines.112,113
The standard treatment for TB-HIV co-infection is 2HRZE/4HR. Resistance to anti-TB drugs is more common and is managed in the same way as in immunocompetent patients. Some interaction between rifampicin and protease inhibitors and some non-nucleoside reverse transcriptase inhibitors may occur.115 There are various options for these cases, including completing anti-TB treatment before starting antiretrovirals, introducing them after an initial 2-month TB treatment period, or 2–8 weeks after starting TB treatment, depending on CD4+ count. If rifampicin cannot be used, treatment is less effective. Rifabutin can be administered instead, or the isoniazide and ethambutol maintenance phase can be extended to up to 18 months.116
Some patients (16%; 10/61) can develop a paradoxical increase in PE during treatment.117 This is due to the immunological rebound effect: at the start of treatment, an excessive antigen load resulting from rapid mycobacterial lysis is released in the PF. This converges with an improvement in cell immunity, leading to a new hypersensitivity reaction.118
There is some controversy regarding whether the anti-inflammatory action of oral corticosteroids can speed up fluid reabsorption, thus preventing pleural adhesions. No differences in the development of residual pleural thickening, adhesions or loss of lung function were observed in 3 clinical trials.119–121 However, one of these studies reported fewer events and speedier resolution of PE in patients taking corticosteroids on days of fever.121 In a TBPE-HIV series, administration of corticosteroids was associated with a greater risk of Kaposi's sarcoma.122 A Cochrane review concluded that there is no evidence to support the use of corticosteroids in these patients.123
Routine PE drainage is not generally recomended124 unless a large PE is causing dyspnea.119 If TBPE is loculated, fibrinolytics will accelerate PE resolution and reduce residual pleural thickening.125,126 This thickening could be associated with the magnitude of the initial change in pH, glucose, and tumor necrosis factor-α (TNF-α) levels.127
ConclusionsThe incidence of TB and hence TBPE is falling in Spain. In 2009, the incidence of TBPE in Galicia fell by half with respect to 2000. It primarily affects young patients, mainly between 15 and 44 years of age, and almost two thirds of cases occur in men. Clinical presentation varies depending on the population in question. Achieving a definitive diagnosis can be problematic, and several different PF biomarkers may be determined. The correlation between ADA and lymphocyte levels can be useful for diagnosis. TBPE prevalence affects the positive and negative predictive values of ADA, and treatment is the same as for any TB. The addition of corticosteroids does not seem advisable, and in the case of large effusions, chest drainage may give speedier relief from symptoms.
FundingNo funding was received for this paper.
Authors ContributionFerreiro L: Author and editor. Conception and design. Final approval of the manuscript. San José E: Co-author and editor. Final approval of the manuscript. Valdés L: Editor and author. Conception and design. Final approval of the manuscript.
Conflict of InterestThe authors have no conflict of interest.
Please cite this article as: Ferreiro L, San José E, Valdés L. Derrame pleural tuberculoso. Arch Bronconeumol. 2014;50:435–443.