Dyspnea is a multidimensional symptom, but this multidimensionality is not considered in most dyspnea questionnaires. The Dyspnea-12 takes a multidimensional approach to the assessment of dyspnea, specifically the sensory and the affective response. The objective of this study was to translate into Spanish and validate the Dyspnea-12 questionnaire.
MethodsThe original English version of the Dyspnea-12 questionnaire was translated into Spanish and backtranslated to analyze its equivalence. Comprehension of the text was verified by analyzing the responses of 10 patients. Reliability and validation of the questionnaire were studied in an independent group of COPD patients attending the pulmonology clinics of Hospital Universitario Marqués de Valdecilla, diagnosed and categorized according to GOLD guidelines.
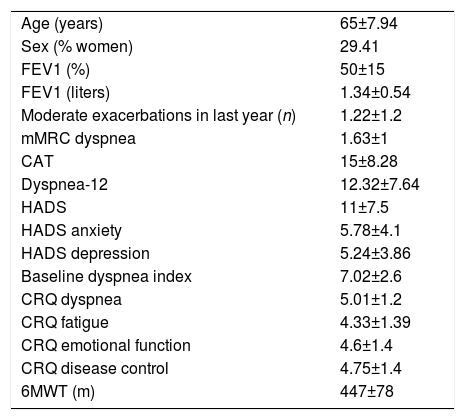
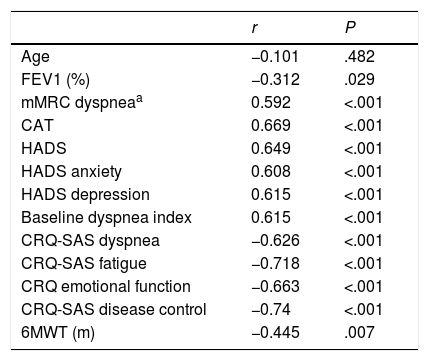
ResultsThe mean age of the group (n=51) was 65 years and mean FEV1 was 50%. All patients understood all questions of the translated version of Dyspnea-12. Internal consistency of the questionnaire was α=0.937 and intraclass correlation coefficient was =0.969; P<.001. Statistically significant correlations were found with HADS (anxiety r=0.608 and depression r=0.615), mMRC dyspnea (r=0.592), 6MWT (r=–0.445), FEV1 (r=–0.312), all dimensions of CRQ-SAS (dyspnea r=–0.626; fatigue r=–0.718; emotional function r=–0.663; mastery r=–0.740), CAT (r=0.669), and baseline dyspnea index (r=–0.615). Dyspnea-12 scores were 10.32 points higher in symptomatic GOLD groups (B and D) (P<.001).
ConclusionThe Spanish version of Dyspnea-12 is a valid and reliable instrument to study the multidimensional nature of dyspnea.
La disnea es un síntoma con un componente multidimensional, aunque las herramientas que se utilizan habitualmente para evaluarla no tienen en cuenta esta faceta. El cuestionario Disnea-12 valora la multidimensionalidad de la disnea, específicamente las dimensiones afectiva y sensorial. El objetivo de este estudio es validar el cuestionario Disnea-12 al español.
MétodosSe realizó una traducción del original en inglés al español y del español al inglés para verificar la equivalencia del texto. Posteriormente se verificó la comprensión del texto tras pasárselo a 10 pacientes. La fiabilidad y la validez del cuestionario se estudiaron en un grupo independiente de EPOC diagnosticados y clasificados por las guías GOLD de las consultas externas de neumología del Hospital Universitario Marqués de Valdecilla.
ResultadosEl grupo (n=51) tenía una media de edad de 65 años y un FEV1 medio del 50%. Todos los pacientes entendieron las preguntas del cuestionario. El instrumento presentó consistencia interna de α=0,937 y un coeficiente de correlación intraclase: 0,969; p<0,001. Se encontraron correlaciones estadísticamente significativas con las puntuaciones del HAD (HADansiedad r=0,608 y HADdepresión r=0,615), disnea de la mMRC (r=0,592), T6MM (r=–0,445), FEV1 (r=–0,312), las 4 dimensiones de CRQ-SAS (disnea r=–0,626; fatiga r=–0,718; función emocional r=–0,663; control de enfermedad r=–0,740), el CAT (r=0,669) y el índice de disnea basal (r=–0,615). Los grupos GOLD más sintomáticos (B y D) presentaron una puntuación 10,32 puntos mayor en en el Disnea-12 (p<0,001).
ConclusiónEl cuestionario Disnea-12 es un instrumento válido y fiable para evaluar la disnea de forma multidimensional.
Dyspnea is defined as a subjective experience consisting of different qualitative sensations that can vary in intensity.1 It is a key symptom in patients with COPD, and is used to assess the severity and prognosis of the disease.2 However, dyspnea is typically a complex, subjective symptom in which environmental, physiological and psychological factors play a part.1 Characteristic features of dyspnea include a sensory component (central chest pain, air hunger, fatigue, difficulty in achieving a full inspiration),3 and an affective component (such as a sensation of distress, sadness or fear presented by some dyspnea patients).4 Some diseases are more often associated with either a specific sensory or affective component: for example, patients with bronchospasm tend to describe their dyspnea as chest tightness,5 while patients with dynamic lung hyperinflation6 often seen in emphysema patients7 more often report sensations such as increased work of breathing.
Dyspnea is routinely measured in clinical practice on two different types of scale. Scales such as the Borg dyspnea scale,8 which aim to quantify perceptions, are generally used to assess dyspnea at rest or in response to certain stimuli, such as physical exercise. The Borg scale describes the intensity of the dyspnea without taking into account the qualitative aspects of how the symptom is experienced. In contrast, tools such as the Medical Research Council (MRC) scale and the modified version of the MRC (mMRC),9 indirectly assess dyspnea according to the patient's ability to perform certain activities, without taking into account the sensory experience or the affective component of the symptom.
The Dyspnea-12 (Fig. 1), originally developed in the UK, is a short questionnaire, initially validated in COPD and heart failure,10 and later in several cardiopulmonary diseases (interstitial lung disease,11 asthma,12 pulmonary hypertension,13 cancer,14 tuberculosis, and bronchiectasis15). Dyspnea-12 takes into account both sensory and affective factors that might play a role in dyspnea, and which could help clinicians to understand the various aspects of the disease. Each item in the questionnaire is scored from 0, if the symptom is mild, to 3, if it is severe, and the overall score is the sum total of all score of all items. Six of the questions refer to sensory aspects and 6 to the affective aspects of dyspnea. The total score ranges from 0 to 36, with 36 being the highest severity possible and 0 the lowest.
Our aim was to produce a Spanish version of the Dyspnea-12 and to determine the reliability and validity of the translation, in order to provide a tool that could be used to measure the degree of dyspnea in our own COPD patients, while taking into account the different components of this symptom.
MethodsInternationally accepted techniques,16,17 similar to those used for the translation of this questionnaire into other languages, were used for the translation and validation of the questionnaire.18 The study protocol was evaluated and approved by the Clinical Research Ethics Committee of Cantabria.
PatientsSpanish-speaking adult patients previously diagnosed with COPD, according to the Global Initiative for Chronic Obstructive Lung (GOLD) criteria,19 seen consecutively between October and December 2016 in the Hospital Universitario Marqués de Valdecilla, were selected for this study. Patients with cognitive impairment or sensory disorders that might prevent them from understanding of the questions were excluded. All patients signed informed consent before completing the questionnaire.
TranslationInitially, the questionnaire was translated twice, by two independent qualified translators. Two bilingual pulmonologists and two Spanish-speaking nurses, experts in respiratory tract diseases, subsequently evaluated the relevance and comprehensibility of the translated items.
After this process, a single version of the questionnaire was created that was then back-translated into English by a qualified translator, who verified that the translation was equivalent to the original. Any discrepancies were resolved by consensus. Finally, the translated questionnaire was read to 10 COPD patients who had signed informed consent; 8 of these 10 patients had completed primary education only. Each translated item of the Dyspnea-12 questionnaire was read to them, and they were asked to explain the meaning in their own words, to verify that they clearly understood the questions. All patients included in the study correctly understood the translation of the questionnaire. Patients were also asked if they thought the questionnaire should be modified in any way, but none of them requested any changes.
Statistical AnalysisThe sample size was estimated on the basis of the number of patients involved in the initial validation.10 The statistical analysis was performed using the SPSS package, version 20.0 (IBM). The statistical techniques used to estimate the reliability and validity of the questionnaire are described below.
Questionnaire ReliabilityThe internal consistency of the questionnaire was measured using Cronbach's alpha. The concordance between the results was evaluated using the intraclass correlation coefficient, evaluating the total score of the Dyspnea-12 questionnaires obtained in the initial assessment and 30min later. Concordance was reassessed 15 days later using the same method employed in the 30-minute re-evaluation.
Questionnaire ValidityThe same variables were used as in the original study (mMRC, HADS, FEV1 and the 6-minute walk test). Questionnaire validity was assessed using Pearson's correlation test for parametric variables (HADS, FEV1 and the 6-minute walk test), and the Spearman test for non-parametric variables (mMRC dyspnea scale).
We also studied the correlation between the questionnaire and other variables that had not been evaluated in the initial validation, such as different scores on the Chronic Respiratory Disease Questionnaire Self-Administered Standardized (McMaster University) (CRQ-SAS), the COPD Assessment Test (CAT) and the baseline dyspnea index. As these were all parametric variables, they were evaluated using Pearson's correlation test. The score of the Dyspnea-12 questionnaire was also compared among COPD patients considered symptomatic according to the GOLD classification19 (GOLD 2017, B and D) and those considered less symptomatic (GOLD A and C).
ResultsThe Spanish translation of the Dyspnea-12 questionnaire is shown in Fig. 1.
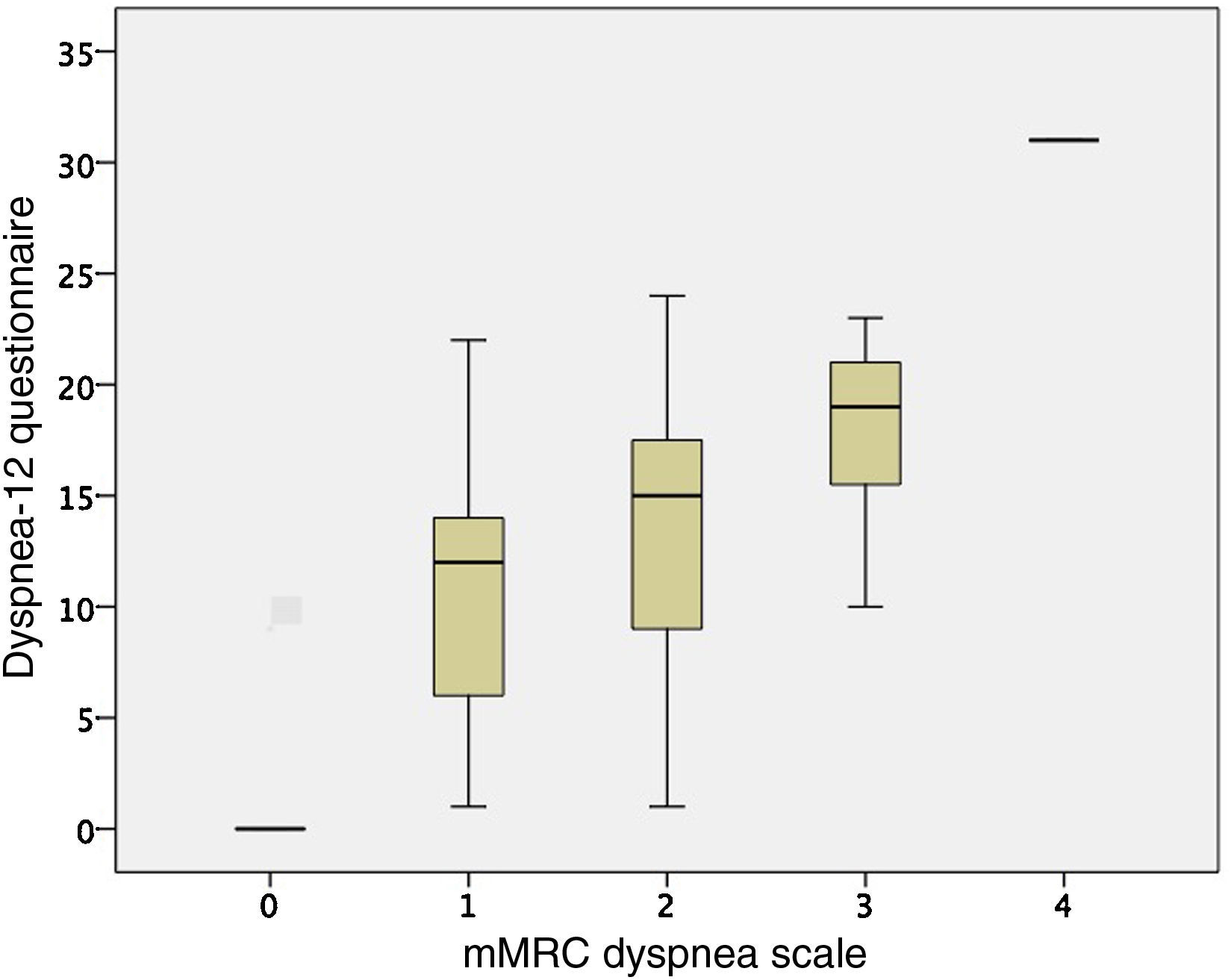
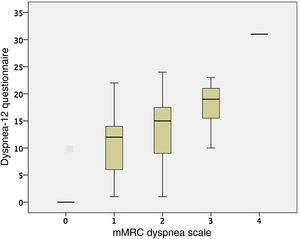
CohortCohort values are expressed as mean (±standard deviation). In total, 51 COPD patients were recruited, 15 of whom were women (29.41%), aged 65 (8 years), with FEV1 50% (15%); they walked 447 meters (78m) on the 6-minute walk test, their CAT score was 15 points (8.28 points), and 15 patients (29.41%) had 2 or more exacerbations in the last year. mMRC dyspnea grades were similar between study patients (5 grade 0, 22 grade 1, 12 grade 2, 11 grade 3 and 1 grade 4) with regard to GOLD spirometric obstruction grade (3 grade I, 24 grade II, 17 grade III, and 7 grade IV), and GOLD groups (7 GOLD A, 26 GOLD B, 4 GOLD C and 14 GOLD D). The Dyspnea-12 questionnaire score was 12.32 points (7.64 points). The most important variables of the study patient cohort are shown in Table 1.
Study Variables.
| Age (years) | 65±7.94 |
| Sex (% women) | 29.41 |
| FEV1 (%) | 50±15 |
| FEV1 (liters) | 1.34±0.54 |
| Moderate exacerbations in last year (n) | 1.22±1.2 |
| mMRC dyspnea | 1.63±1 |
| CAT | 15±8.28 |
| Dyspnea-12 | 12.32±7.64 |
| HADS | 11±7.5 |
| HADS anxiety | 5.78±4.1 |
| HADS depression | 5.24±3.86 |
| Baseline dyspnea index | 7.02±2.6 |
| CRQ dyspnea | 5.01±1.2 |
| CRQ fatigue | 4.33±1.39 |
| CRQ emotional function | 4.6±1.4 |
| CRQ disease control | 4.75±1.4 |
| 6MWT (m) | 447±78 |
6MWT: 6-minute walk test; CAT: COPD Assessment Test; CRQ: Chronic Respiratory Disease Questionnaire Self-Administered Standardized, McMaster University; FEV1: forced expiratory volume in 1 second; HADS: Hospital Anxiety and Depression Scale; mMRC: modified Medical Research Council.
The Spanish version of the Dyspnea-12 showed good internal consistency (α=0.937) and a good intraclass correlation coefficient (0.969; P<.001) for the test repeated after 30minutes.
The intraclass correlation coefficient 15 days after the initial assessment was 0.964; P<.001).
ValidityFor the other variables examined, the Dyspnea-12 correlated with the HADS scores (HADS anxiety r=0.608; P<.001, and HAD depression r=0.615; P<.001), and the mMRC dyspnea grade (r=0.592; P<.001). The comparison between the mMRC dyspnea grade and Dyspnea-12 scores are shown in Fig. 2. Correlation was also observed with the distance walked on the 6-minute walk test (r=–0.445; P=.007), and FEV1 (r=–0.312; P=.029). These results are similar to those obtained in the initial questionnaire assessment.10
Correlation was observed with the 4 dimensions of CRQ-SAS (dyspnea P<.001, r=–0.626; fatigue P<.001, r=–0.718; emotional function P<.001, r=–0.663; disease control P<.001, r=–0.740), with the CAT (P<.001, r=0.669), and with the baseline dyspnea index (P<.001 r=–0.615). These results and other significant correlations are presented in Table 2.
Correlations With the Dyspnea-12 Questionnaire.
| r | P | |
|---|---|---|
| Age | −0.101 | .482 |
| FEV1 (%) | −0.312 | .029 |
| mMRC dyspneaa | 0.592 | <.001 |
| CAT | 0.669 | <.001 |
| HADS | 0.649 | <.001 |
| HADS anxiety | 0.608 | <.001 |
| HADS depression | 0.615 | <.001 |
| Baseline dyspnea index | 0.615 | <.001 |
| CRQ-SAS dyspnea | −0.626 | <.001 |
| CRQ-SAS fatigue | −0.718 | <.001 |
| CRQ emotional function | −0.663 | <.001 |
| CRQ-SAS disease control | −0.74 | <.001 |
| 6MWT (m) | −0.445 | .007 |
6MWT: 6-minute walk test; CAT: COPD Assessment Test; CRQ-SAS, Chronic Respiratory Disease Questionnaire Self-Administered Standardized, McMaster University; FEV1: forced expiratory volume in 1 second; HADS: Hospital Anxiety and Depression Scale; mMRC: modified Medical Research Council.
When the Dyspnea-12 questionnaire scores from the more symptomatic (B and D) (n=39) and the less symptomatic (A and C) (14) (n=11) GOLD groups were compared, a statistically significant difference of 10.32±2.18 points was found in the Dyspnea-12 score (P<.001).
DiscussionThe Dyspnea-12 questionnaire, translated and validated in Spanish, is a valid, reliable instrument for the multidimensional measurement of dyspnea in that language. Until now, dyspnea has been measured indirectly using questionnaires that in reality measured the degree of limitation (e.g., the mMRC questionnaire).9 The Dyspnea-12 is a simple, quick tool that provides a useful evaluation of dyspnea, taking into account how patients experience their dyspnea (sensory score), and how they feel because of it (affective score).
In our study, the instrument was translated using methods similar to those used for the translation of other questionnaires into Spanish, and for the translation of Dyspnea-12 into other languages (such as Arabic).16–18 Healthcare professionals, professional translators, and the patients themselves were involved in the process.
To validate the translation, a very similar cohort to that used in the original validation of the questionnaire was used,10 and we found that the correlations with the reference variables in our study are similar to those of the original study.
Other questionnaires are available for the multidisciplinary evaluation of dyspnea. The Multidimensional Dyspnea Profile,1 for example, was recently compared with the Dyspnea-12,20 and the authors concluded that both tools may be useful for assessing dyspnea in our patients.
The main limitation of our study is that exclusively COPD patients were included. However, this questionnaire has already been validated in Spanish for other cardiorespiratory diseases, such as idiopathic pulmonary fibrosis, pulmonary hypertension, and heart failure, although the initial validation focused on COPD patients.10 Another limitation of the study is its sample size, although the number of patients included is similar to that of the original validation of the questionnaire, and the cohort is heterogeneous, as shown in Table 1 and described in the results section. No sensitivity to change analysis was performed, either for the Spanish version or for any other version of the questionnaire. Patients included in the study are from the catchment area of the Hospital Universitario Marqués de Valdecilla, in Santander (Spain), and this may represent a limitation, since the sociodemographic characteristics of this area may differ from those of other regions of Spain. Despite these limitations, we believe that our translation of the questionnaire should be applicable to respiratory patients with a basic command of Spanish.
The routine applicability of this questionnaire in clinical practice has yet to be confirmed, but it is likely to be useful as a simple tool for distinguishing organic dyspnea from functional dyspnea, improving the classification of dyspnea among our patients, and better understanding both the mechanism of our patients’ dyspnea, and the results of certain functional tests.21
Finally, we can conclude we have translated and validated a promising tool for the multidimensional study of dyspnea.
FundingSource of funding: the IDIVAL Health Research Institute.
Conflict of InterestsThe authors state that they have no conflict of interests.
We thank Marta Suárez for her help in recruiting patients in this study.
Please cite this article as: Amado Diago CA, Puente Maestu L, Abascal Bolado B, Agüero Calvo J, Hernando Hernando M, Puente Bats I, et al. Traducción y validación del cuestionario multidimensional Disnea-12. Arch Bronconeumol. 2018;54:74–78.